



 下载产品说明书
下载产品说明书 用小程序,查商品更便捷
用小程序,查商品更便捷



 收藏
收藏
 对比
对比 咨询
咨询
Product Overview
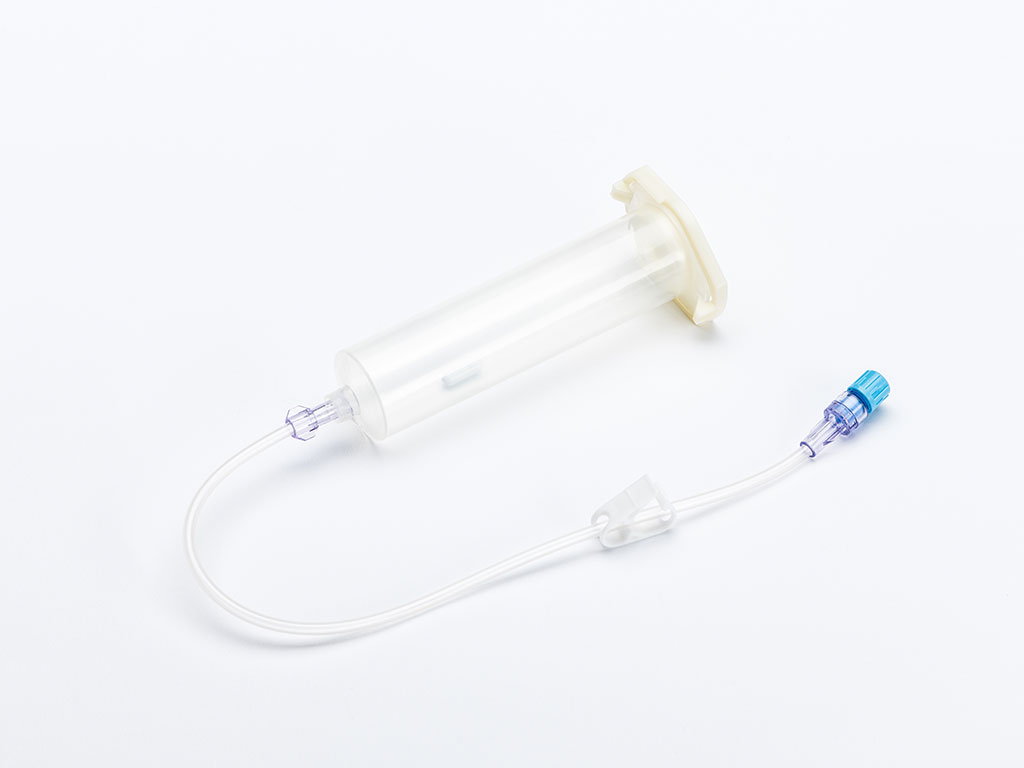
Large-Scale Transfection Using the 4D-Nucleofector® LV Unit - Experience the latest functional unit for the 4D-Nucleofector® System which expands our proven system to larger-scale transfection.
The LV Unit allows for closed, scalable transfection of larger cell numbers in the range of 1x107 to 1x109 cells. Transfection protocols can be established in smaller scale using the X Unit and subsequently transferred to the LV Unit without the need for re-optimization. Transferability has been tested for various cell types, including human T cells, CHO-S, HEK293-S, or K-562.
Accompanied by GMP grade TheraPEAK® Consumables, a 21CFR part 11 compliant software and IQOQ services the LV Unit is suited to quickly translate research results into a GMP compliant manufacturing process.
There are cell type-specific Optimized Protocols or recommendations available in our knowledge database. In these Optimized Protocols the best Nucleofection conditions are indicated. In addition, we share our experience and knowledge for treatment of individual cell types.
Technical Specifications
Dimensions: (w x h x d): 24.5×20.7×28 cm / 9.7×4.1×11.0
Weight: 5.7 kg (12.5 lb)
Power: 100 - 240 VAC
Frequency: 50 - 60 Hz
Consumption: 0 VA (power off) / 20 VA (idle) / 140 VA (busy - during transfection)
Fuse: T 2.5A, L250V
Operating temperature: +15°C up to +40°C (59°F - 104°F)
Environment non condensing voltage limitation: 1500 V
Current limitation: 100 A
Quality statement:
We are applying ISO 9001:2015 standards for the design, manufacturing and testing of Nucleofector® Devices since 2004.
* TheraPEAK® Nucleofector® Products are produced according to applicable GMP raw material standards and are intended to support GMP manufacturing. TheraPEAK® Nucleofector® Solutions are manufactured at a GMP certified site. TheraPEAK® Nucleofector® Cartridges and Reservoirs are produced at manufacturing sites with an ISO13485 certified quality management system. TheraPEAK® Nucleofector® Products are not intended for in vivo and/or diagnostic purposes. All other Nucleofector® Products are for research use only.
Request More Info
Benefits
- Closed system: Sterile Nucleofection of up to 109 cells
- Real scalability: Optimization in small scale
- Established protocols: Benefit from 700+ optimized cell types
- Simple handling: Minimal training needs
- 4D-Nucleofector® LogWare: Optional operation via 21CFR part11 compliant software
- Fit for purpose: Consumables for research use and GMP manufacturing*
Applications
- Ex-vivo modification of human primary cells for cell therapy applications (e.g. genome editing, generation of CAR-T cells)
- Transient production of potential therapeutic proteins or antibodies for construct screening
- Generation of large numbers of transiently modified primary cells for cell-based assays





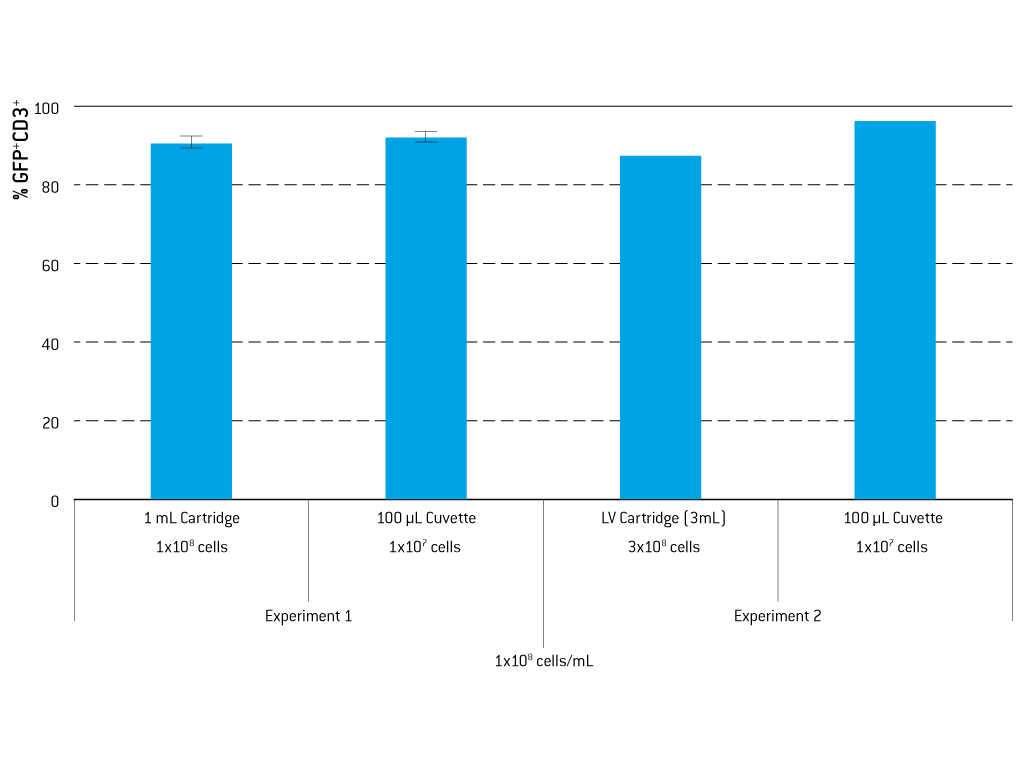





 危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)
危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)  营业执照(三证合一)
营业执照(三证合一)