


 下载产品说明书
下载产品说明书 下载SDS
下载SDS 用小程序,查商品更便捷
用小程序,查商品更便捷



 收藏
收藏
 对比
对比 咨询
咨询







参考图片
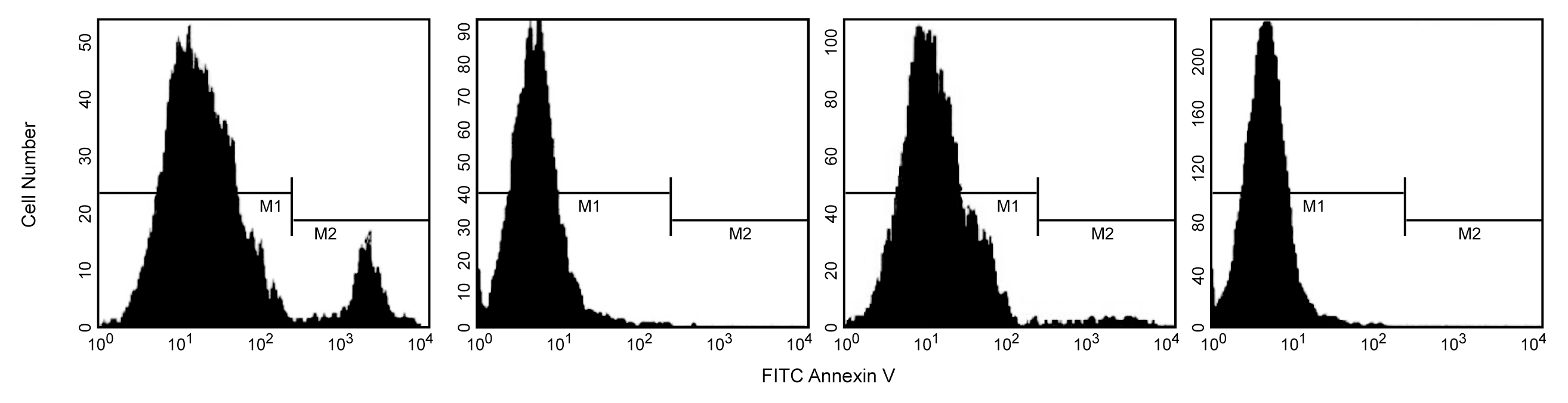
Flow cytometric analysis of Annexin V staining and blocking. Jurkat T cells were induced to undergo apoptosis by treatment with anti-Fas antibody, clone DX2 (Cat. No. 555670) and Protein G for three hours (first & second panels). Other cultures were left untreated (third & fourth panels). Cells were incubated with FITC Annexin V (first & third panels) or with recombinant Annexin V to block Annexin V binding sites, and then with FITC Annexin V (second & fourth panels). After a three hour treatment with Fas mAb, a population of cells was FITC Annexin V positive (first panel, M2 gate). FITC Annexin V staining was blocked when cells were first incubated with purified recombinant Annexin V (second panel). As expected, the cell population that was not treated with Fas mAb were primarily Annexin V negative (third panel). The small number of Annexin V positive cells in the untreated population likely represents a basal level of apoptosis. This was blocked when cells were first incubated with purified recombinant Annexin V (fourth panel). The M1 and M2 gates demarcate FITC Annexin V negative and positive populations, respectively. The addition of Protein G enhances the ability of DX2 to induce apoptosis, presumably by crosslinking the Fas receptor.
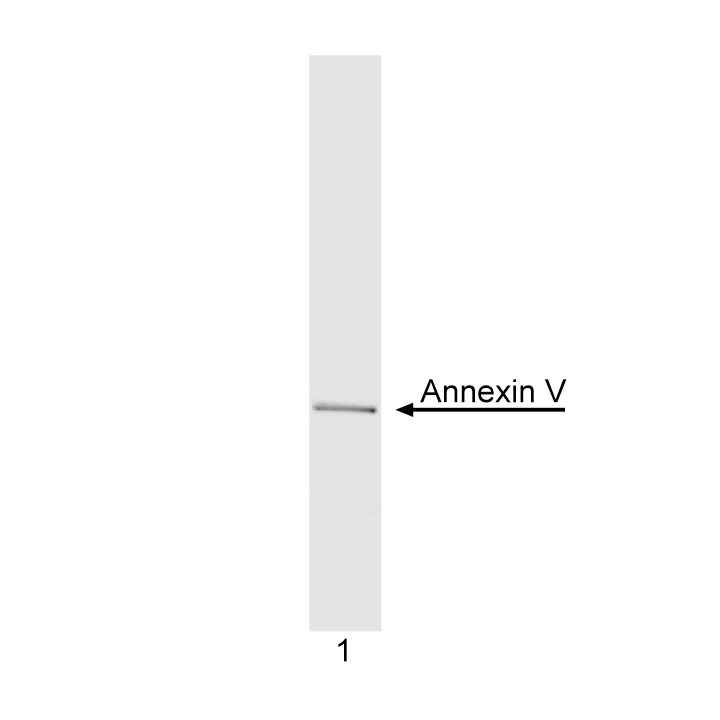
Analysis of purified recombinant Annexin V (1 µg) by SDS/PAGE. Gel was stained with Commasie Blue.
Flow cytometric analysis of Annexin V staining and blocking. Jurkat T cells were induced to undergo apoptosis by treatment with anti-Fas antibody, clone DX2 (Cat. No. 555670) and Protein G for three hours (first & second panels). Other cultures were left untreated (third & fourth panels). Cells were incubated with FITC Annexin V (first & third panels) or with recombinant Annexin V to block Annexin V binding sites, and then with FITC Annexin V (second & fourth panels). After a three hour treatment with Fas mAb, a population of cells was FITC Annexin V positive (first panel, M2 gate). FITC Annexin V staining was blocked when cells were first incubated with purified recombinant Annexin V (second panel). As expected, the cell population that was not treated with Fas mAb were primarily Annexin V negative (third panel). The small number of Annexin V positive cells in the untreated population likely represents a basal level of apoptosis. This was blocked when cells were first incubated with purified recombinant Annexin V (fourth panel). The M1 and M2 gates demarcate FITC Annexin V negative and positive populations, respectively. The addition of Protein G enhances the ability of DX2 to induce apoptosis, presumably by crosslinking the Fas receptor.
Analysis of purified recombinant Annexin V (1 µg) by SDS/PAGE. Gel was stained with Commasie Blue.






 危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)
危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)  营业执照(三证合一)
营业执照(三证合一)