FDA解读临床Biomarkers,和部分热点Biomarkers
时间:2017-03-19 06:18:34 浏览次数:1322
|
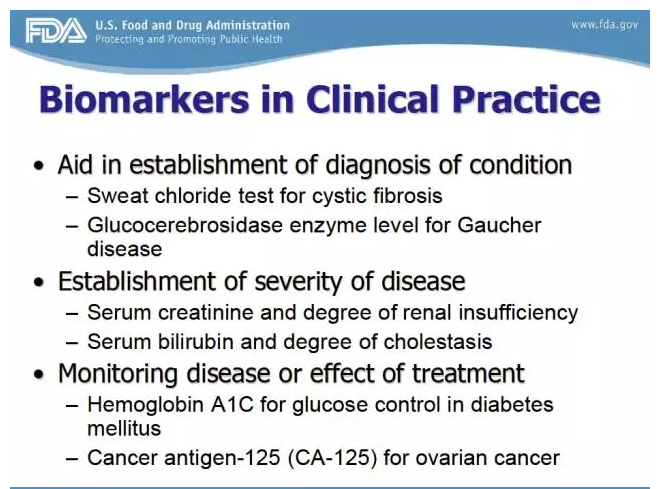
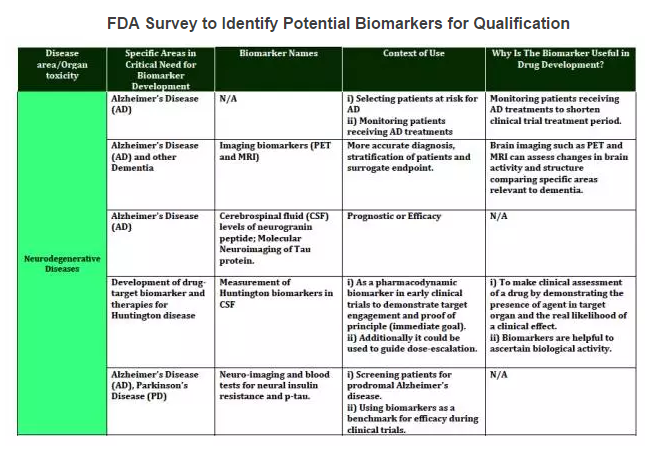
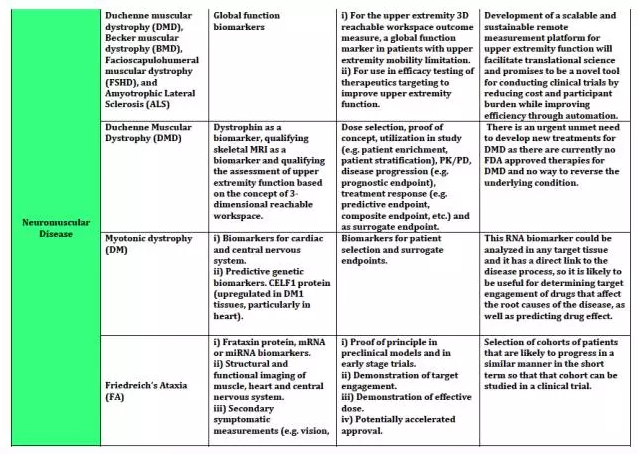
The Biomarker Qualification Program was established to support the Center for Drug Evaluation and Research’s (CDER's) work with external stakeholders to develop biomarkers that aid in the drug development process. Through the FDA’s Biomarker Qualification Program, you may request regulatory qualification of a biomarker for a particular context of use in drug development. Biomarkers can be used in a variety of settings, including basic research, drug development, and clinical practice. The Biomarker Qualification Program focuses on biomarkers used in drug development. Once a biomarker is qualified, it can be used in any drug development program under the context for which it obtained qualification.
The Biomarker Qualification Program is one of the Drug Development Tools (DDT) Qualification Programs created by CDER to provide a framework for development and regulatory acceptance of scientific tools for use in drug development programs.
Why qualify a biomarker through the CDER Biomarker Qualification Program?
A qualified biomarker can be used in multiple drug development programs without a need for CDER to reconfirm the suitability of the biomarker’s qualified context of use and has the potential to advance public health by streamlining the drug development paradigm.
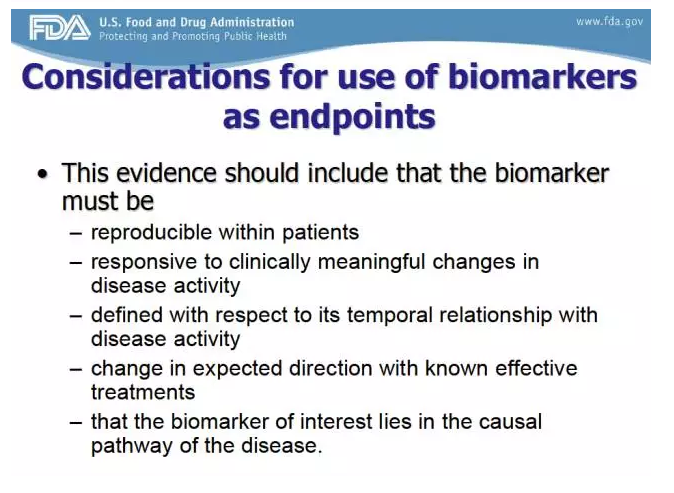
What is the process to qualify a biomarker?
The biomarker qualification process consists of three stages: (1) Initiation, (2) Consultation and Advice, and (3) Review.
          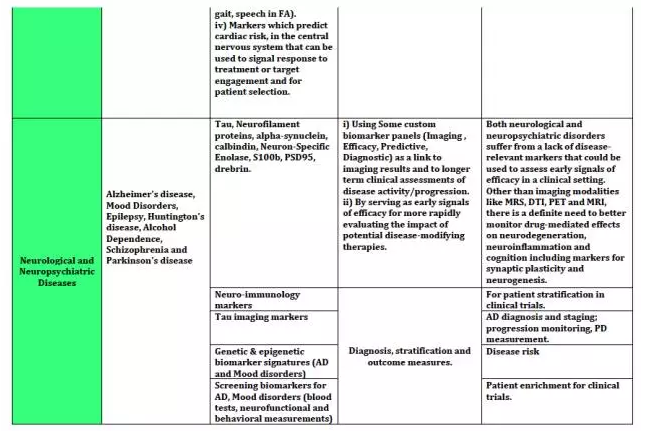 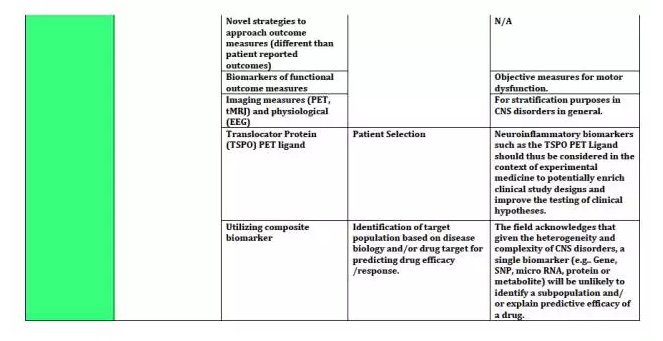 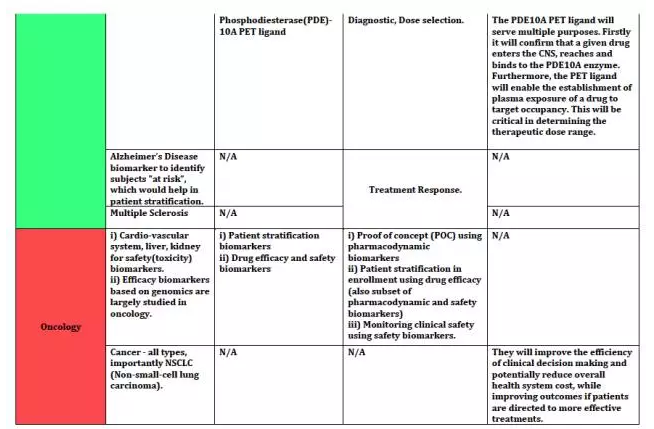 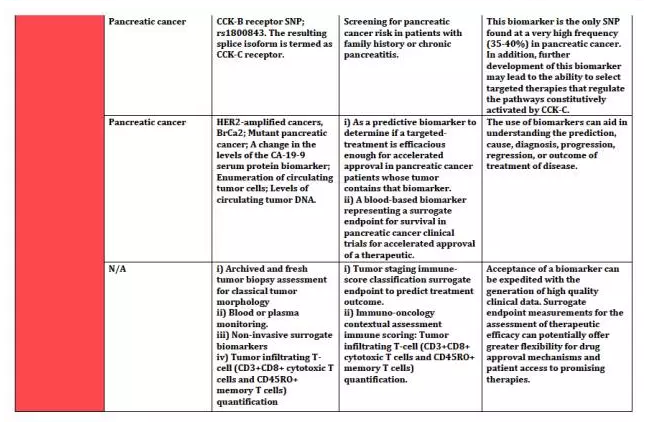       上海优宁维生物科技股份有限公司
咨询邮箱:equip@univ-bio.com 微信公众平台:优宁维科学仪器
|
上海优宁维生物科技股份有限公司
试剂 | 耗材 | 仪器 | 软件 | 定制 | 实验服务 | 供应链
免费热线:4008-168-068
咨询邮箱:info@univ-bio.com
订购商城:www.univ-bio.com
微信公众平台:优宁维抗体专家,欢迎关注!
小优博士(小程序):5大课堂, 让你的科研不再难!









 危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)
危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)  营业执照(三证合一)
营业执照(三证合一)