


 下载产品说明书
下载产品说明书 下载SDS
下载SDS 用小程序,查商品更便捷
用小程序,查商品更便捷



 收藏
收藏
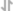 对比
对比 咨询
咨询Scientific Data
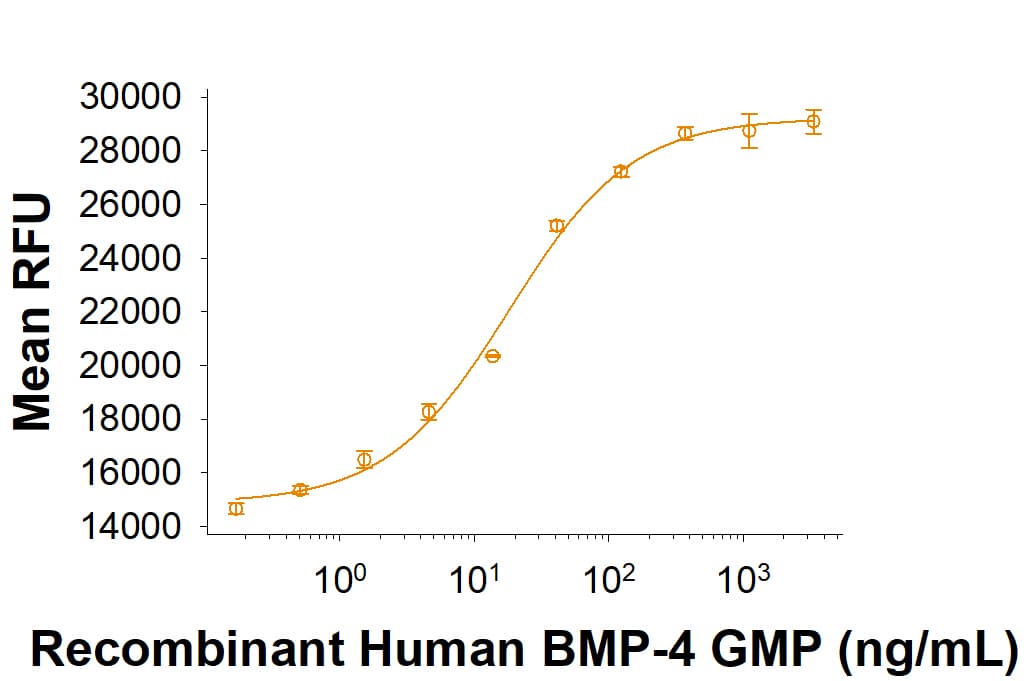
 View Larger
View LargerGMP-grade Recombinant Human BMP-4 (Catalog # 314E-GMP) induces BMP responsive SEAP reporter activity in HEK293 human embryonic kidney cells. The ED50 for this effect is 3-21 ng/mL.
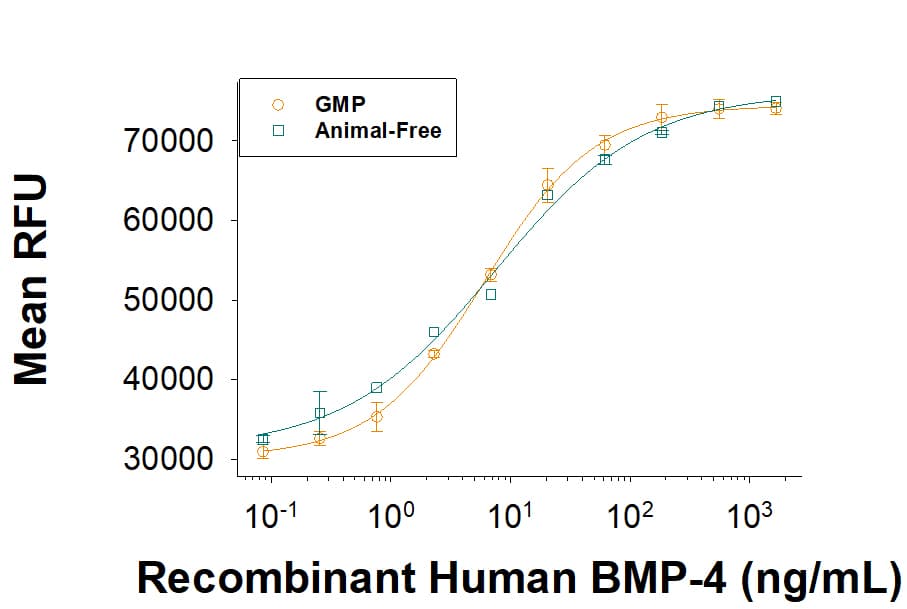
 View Larger
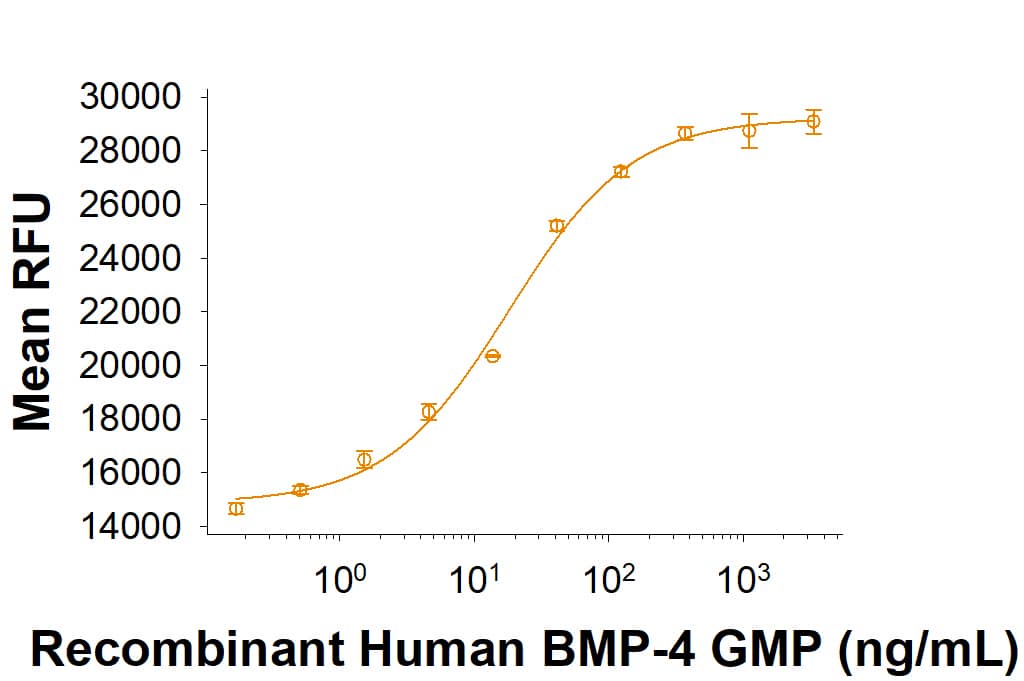
View LargerEquivalent bioactivity of GMP (Catalog # 314E-GMP) and Animal-Free (AFL314E) grades of Recombinant Human BMP-4 as measured by its ability to induce BMP responsive SEAP reporter activity in HEK293 human embryonic kidney cells (orange and green respectively).
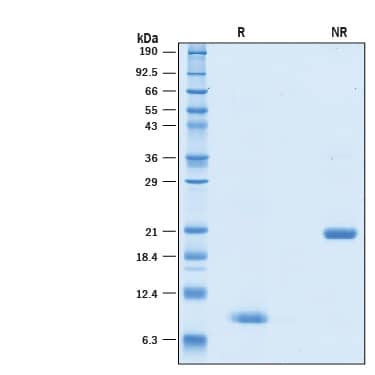 View Larger
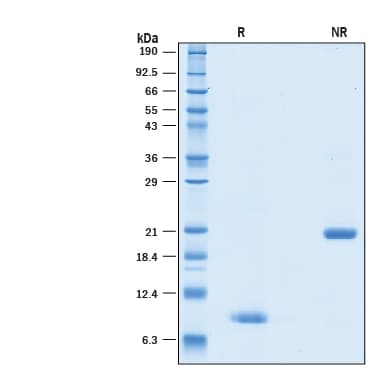
View Larger2 μg/lane of GMP-grade Recombinant Human BMP-4 Protein (314E-GMP) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 8-9 kDa and 20-22 kDa, respectively.
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
314E-GMP
| Formulation | Lyophilized from a 0.2 μm filtered solution in Acetonitrile and TFA. |
| Reconstitution | Reconstitute at 50-200 μg/mL in sterile 4 mM HCl. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Recombinant Human BMP-4 GMP Protein, CF Summary
Product Specifications
Lys303-Arg408
Produced in an animal-free laboratory. Manufactured and tested under cGMP guidelines
Analysis

Background: BMP-4
Bone morphogenetic protein 4 (BMP-4) is a TGF-beta superfamily ligand that is widely expressed from early embryogenesis through adulthood. It plays an important role in mesenchyme formation, epidermal determination, suppression of neural induction, the development of multiple organs, and tissue repair (1-5). It is an integral part of many stem cell differentiation pathways, including lung tissue, (6), adipogenesis (7) and osteogenesis (8, 9). The human BMP-4 precursor contains a 273 amino acid (aa) propeptide and a 116 amino acid (aa) mature protein (10). Processing of the propeptide by furin or proprotein convertase 6 enables the formation of the mature disulfide-linked homodimeric BMP-4 and facilitates its secretion. Similar intracellular processes may lead to the formation and recreation of BMP4/BMP7 disulfide-linked heterodimer (11-13). Mature human and mouse BMP-4 share 98% aa sequence identity. Human BMP-4 shares 85% aa sequence identity with human BMP-2 and less than 50% with other human BMPs. In Xenopus, BMP-4 dimers provide ventralizing signals for existing mesoderm (14). BMP-4 signals through tetrameric complexes composed of type I (primarily Activin RIA or BMPR-IA) and type II (primarily Activin RIIA or BMPR-II) receptors (15, 16). The bioavailability of BMP-4 is regulated by its interaction with multiple proteins and glycosaminoglycans (17-19).
- Zhang, P. et al. (2008) Blood 111:1933.
- Gambaro, K. et al. (2006) Cell Death Differ. 13:1075.
- Simic, P. and S. Vukicevic (2005) Cytokine Growth Factor Rev. 16:299.
- Sadlon, T.J. et al. (2004) Stem Cells 22:457.
- Frank, D.B. et al. (2005) Circ. Res. 97:496.
- Lee, J-H. et al. (2014) Cell. 156:440.
- Tang, QQ. et Lane MD. (2012) Annu Rev. Biochem. 81:715.
- Urist, MR. (1965). Science. 150:893.
- Bandyopadhyay, A. et al. (2006) Plos Genetics. 2:e216.
- Wozney, J. et al. (1988) Science 242:1528.
- Cui, Y. et al. (1998) EMBO J. 17:4735.
- Cui, Y. et al. (2001) Genes Dev. 15:2797.
- Aono, A. et al. (1995) Biochem. Biophys. Res. Commun. 210:670.
- Nishimatsu, S. and G.H. Thomsen (1998) Mech. Dev. 74:75.
- Chen, D. et al. (2004) Growth Factors 22:233.
- Lavery, K. et al. (2008) J. Biol. Chem. April 24 epub.
- Rosen, V. (2006) Ann. N.Y. Acad. Sci. 1068:19.
- Jones, C.M. and J.C. Smith (1998) Dev. Biol. 194:12.
- Takada, T. et al. (2003) J. Biol. Chem. 278:43229.






 危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)
危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)  营业执照(三证合一)
营业执照(三证合一)