



 下载产品说明书
下载产品说明书 下载SDS
下载SDS 用小程序,查商品更便捷
用小程序,查商品更便捷



 收藏
收藏
 对比
对比 咨询
咨询Scientific Data
 View Larger
View LargerRecombinant human M-CSF (Catalog # 216-MC) has a molecular weight (MW) of 39.2 kDa as analyzed by SEC-MALS, suggesting that this protein is a homodimer. MW may differ from predicted MW due to post-translational modifications (PTMs) present (i.e. Glycosylation).
 View Larger
View LargerRecombinant Human M-CSF (Catalog # 216-MC) stimulates cell proliferation of the M‑NFS‑60 mouse myelogenous leukemia lymphoblast cell line in a dose-dependent manner. The ED50 for this effect is 0.5-1.5 ng/mL.
 View Larger
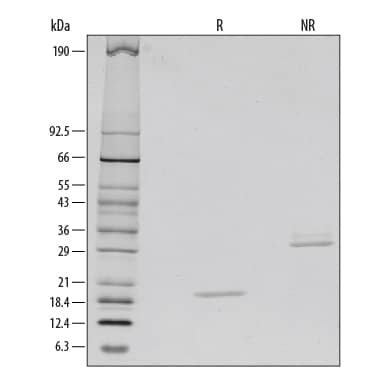
View Larger1 μg/lane of Recombinant Human M-CSF was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by silver staining, showing bands at 19 kDa and 35 kDa, respectively.
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
216-MC
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with BSA as a carrier protein. |
| Reconstitution | Reconstitute at 50-500 μg/mL in sterile PBS containing at least 0.1% human or bovine serum albumin. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
216-MC/CF
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS. |
| Reconstitution | Reconstitute at 50-500 μg/mL in sterile PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Recombinant Human M-CSF Protein Summary
Product Specifications
Glu33-Ser190, with an N-terminal Met
Analysis

Background: M-CSF
M-CSF, also known as CSF-1, is a four-alpha -helical-bundle cytokine that is the primary regulator of macrophage survival, proliferation and differentiation (1-3). M-CSF is also essential for the survival and proliferation of osteoclast progenitors (1, 4). M-CSF also primes and enhances macrophage killing of tumor cells and microorganisms, regulates the release of cytokines and other inflammatory modulators from macrophages, and stimulates pinocytosis (2, 3). M-CSF increases during pregnancy to support implantation and growth of the decidua and placenta (5). Sources of M-CSF include fibroblasts, activated macrophages, endometrial secretory epithelium, bone marrow stromal cells and activated endothelial cells (1-5). The M-CSF receptor (c-fms) transduces its pleotropic effects and mediates its endocytosis. M-CSF mRNAs of various sizes occur (3-9). Full length human M-CSF transcripts encode a 522 amino acid (aa) type I transmembrane (TM) protein with a 464 aa extracellular region, a 21 aa TM domain, and a 37 aa cytoplasmic tail that forms a 140 kDa covalent dimer. Differential processing produces two proteolytically cleaved, secreted dimers. One is an N- and O- glycosylated 86 kDa dimer, while the other is modified by both glycosylation and chondroitin-sulfate proteoglycan (PG) to generate a 200 kDa subunit. Although PG-modified M-CSF can circulate, it may be immobilized by attachment to type V collagen (8). Shorter transcripts encode
M‑CSF that lacks cleavage and PG sites and produces an N-glycosylated 68 kDa TM dimer and a slowly produced 44 kDa secreted dimer (7). Although forms may vary in activity and half-life, all contain the N‑terminal 150 aa portion that is necessary and sufficient for interaction with the M-CSF receptor (10, 11). The first 223 aa of mature human M-CSF shares 88%, 86%, 81% and 74% aa identity with corresponding regions of dog, cow, mouse and rat M‑CSF, respectively (12, 13). Human M-CSF is active in the mouse, but mouse M-CSF is reported to be species-specific.
- Pixley, F.J. and E.R. Stanley (2004) Trends Cell Biol. 14:628.
- Chitu, V. and E.R. Stanley (2006) Curr. Opin. Immunol. 18:39.
- Fixe, P. and V. Praloran (1997) Eur. Cytokine Netw. 8:125.
- Ryan, G.R. et al. (2001) Blood 98:74.
- Makrigiannakis, A. et al. (2006) Trends Endocrinol. Metab. 17:178.
- Nandi, S. et al. (2006) Blood 107:786.
- Rettenmier, C.W. and M.F. Roussel (1988) Mol. Cell Biol. 8:5026.
- Suzu, S. et al. (1992) J. Biol. Chem. 267:16812.
- Manos, M.M. (1988) Mol. Cell. Biol. 8:5035.
- Koths, K. (1997) Mol. Reprod. Dev. 46:31.
- Jang, M-H. et al. (2006) J. Immunol. 177:4055.
- Kawasaki, E.S. et al. (1985) Science 230: 291.
- Wong, G.G. et al. (1987) Science 235:1504.









 危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)
危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)  营业执照(三证合一)
营业执照(三证合一)