


 下载产品说明书
下载产品说明书 下载SDS
下载SDS 用小程序,查商品更便捷
用小程序,查商品更便捷



 收藏
收藏
 对比
对比 咨询
咨询Scientific Data
 View Larger
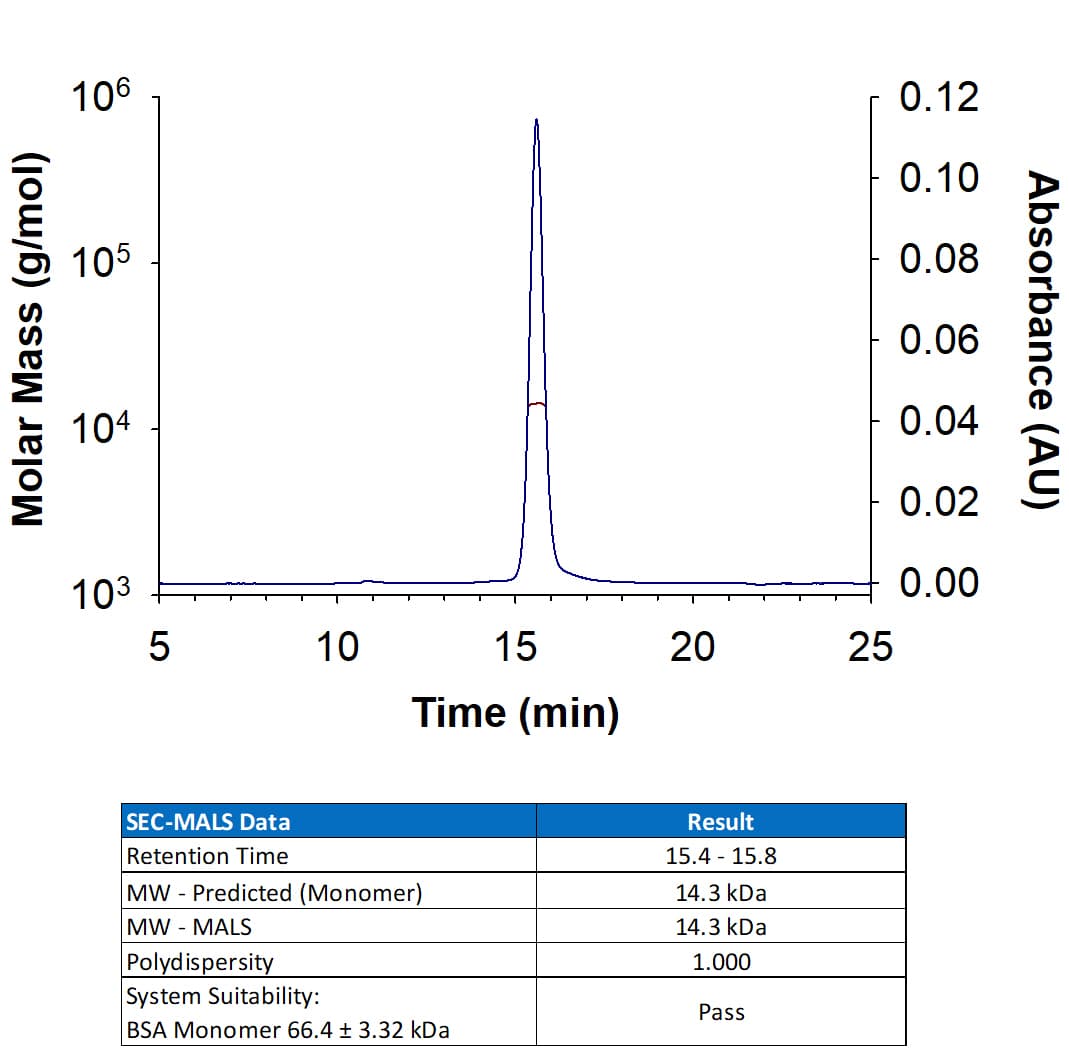
View LargerRecombinant Mouse GM-CSF Protein (Catalog # 415-ML) has a molecular weight (MW) of 14.3 kDa as analyzed by SEC-MALS, suggesting that this protein is a monomer.
 View Larger
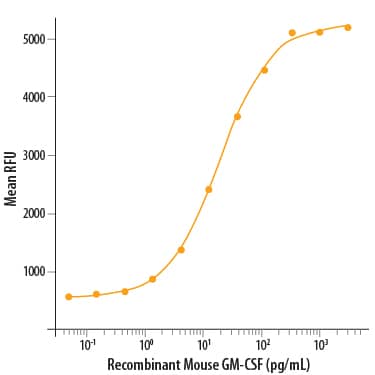
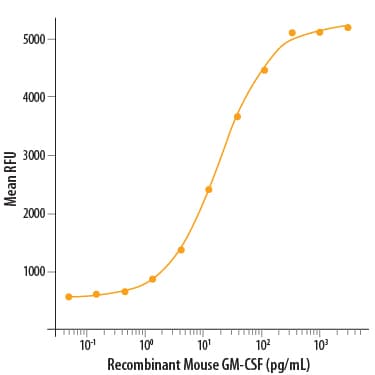
View LargerRecombinant Mouse GM-CSF (Catalog # 415-ML) stimulates cell proliferation of the DA3 mouse myeloma cell line. The ED50 for this effect is 5-30 pg/mL.
 View Larger
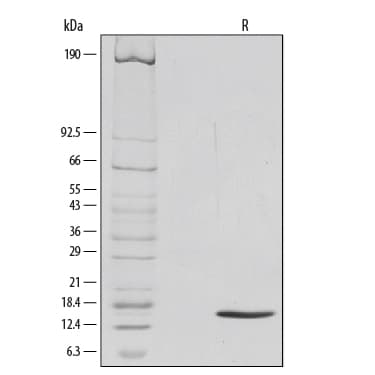
View Larger1 μg/lane of Recombinant Mouse GM-CSF was resolved with SDS-PAGE under reducing (R) conditions and visualized by silver staining, showing a single band at 14 kDa.
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
415-ML
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with BSA as a carrier protein. |
| Reconstitution | Reconstitute at 100 μg/mL in sterile PBS containing at least 0.1% human or bovine serum albumin. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
415-ML/CF
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS. |
| Reconstitution | Reconstitute at 100 μg/mL in sterile PBS. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Recombinant Mouse GM-CSF Protein Summary
Product Specifications
Ala18-Lys141, with an N-terminal Met
Analysis
Background: GM-CSF
GM-CSF was initially characterized as a factor that can support the in vitro colony formation of granulocyte-macrophage progenitors. It is also a growth factor for erythroid, megakaryocyte, and eosinophil progenitors. GM-CSF is produced by a number of different cell types (including T cells, B cells, macrophages, mast cells, endothelial cells, fibroblasts, and adipocytes) in response to cytokine or inflammatory stimuli. On mature hematopoietic cells, GM-CSF is a survival factor for and activates the effector functions of granulocytes, monocytes/macrophages, and eosinophils (1, 2). GM-CSF promotes a Th1 biased immune response, angiogenesis, allergic inflammation, and the development of autoimmunity (3-5). It shows clinical effectiveness in ameliorating chemotherapy-induced neutropenia, and GM-CSF transfected tumor cells are utilized as cancer vaccines (6, 7). The 22 kDa glycosylated GM-CSF, similar to IL-3 and IL-5, is a cytokine with a core of four bundled alpha ‑helices (8-10). Mature mouse GM-CSF shares 49%-54% amino acid sequence identity with canine, feline, human, and porcine GM-CSF and 69% with rat GM‑CSF. GM‑CSF exerts its biological effects through a heterodimeric receptor complex composed of GM-CSF R alpha /CD116 and the signal transducing common beta chain (CD131) which is also a component of the high-affinity receptors for IL-3 and IL-5 (11, 12). In addition, GM-CSF binds a naturally occurring soluble form of GM-CSF R alpha (13). The activity of GM-CSF is species specific between human and mouse. Mouse GM-CSF is only weakly active on rat cells, although rat GM‑CSF is fully active on mouse cells (14, 15).
- Martinez-Moczygemba, M. and D.P. Huston (2003) J. Allergy Clin. Immunol. 112:653.
- Barreda, D.R. et al. (2004) Dev. Comp. Immunol. 28:509.
- Eksioglu, E.A. et al. (2007) Exp. Hematol. 35:1163.
- Cao, Y. (2007) J. Clin. Invest. 117:2362.
- Fleetwood, A.J. et al. (2005) Crit. Rev. Immunol. 25:405.
- Heuser, M. et al. (2007) Semin. Hematol. 44:148.
- Hege, K.M. et al. (2006) Int. Rev. Immunol. 25:321.
- Kaushansky, K. et al. (1992) Biochemistry 31:1881.
- Diederichs, K. et al. (1991) Science 254:1779.
- Gough, N.M. et al. (1984) Nature 309:763.
- Onetto-Pothier, N. et al. (1990) Blood 75:59.
- Hayashida, K. et al. (1990) Proc. Natl. Acad. Sci. 87:9655.
- Pelley, J.L. et al. (2007) Exp. Hematol. 35:1483.
- Oaks, M.K. et al. (1995) J. Interferon Cytokine Res. 15:1095.
- Vandenabeele, P. et al. (1990) Lymphokine Res. 9:381.







 危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)
危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)  营业执照(三证合一)
营业执照(三证合一)