



 下载产品说明书
下载产品说明书 下载SDS
下载SDS 用小程序,查商品更便捷
用小程序,查商品更便捷



 收藏
收藏
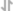 对比
对比 咨询
咨询Scientific Data
 View Larger
View LargerRecombinant Human GM-CSF (Catalog # 7954-GM) has a molecular weight (MW) of 16.3 kDa as analyzed by SEC-MALS, suggesting that this protein is a monomer. MW may differ from predicted MW due to post-translational modifications (PTMs) present (i.e. Glycosylation).
 View Larger
View LargerMeasured in a cell proliferation assay using TF-1 human erythroleukemic cells. The ED50 for this effect is 6-30 pg/mL.
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
7954-GM
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with BSA as a carrier protein. |
| Reconstitution | Reconstitute at 100 μg/mL in PBS containing at least 0.1% human or bovine serum albumin. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
7954-GM/CF
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS. |
| Reconstitution | Reconstitute at 100 μg/mL in PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Recombinant Human GM-CSF (CHO-expressed) Protein Summary
Product Specifications
Ala18-Glu144
Analysis
Background: GM-CSF
GM‑CSF was initially characterized as a factor that can support the in vitro colony formation of granulocyte‑macrophage progenitors. It is also a growth factor for erythroid, megakaryocyte, and eosinophil progenitors. GM‑CSF is produced by a number of different cell types (including T cells, B cells, macrophages, mast cells, endothelial cells, fibroblasts, and adipocytes) in response to cytokine or inflammatory stimuli. On mature hematopoietic cells, GM‑CSF is a survival factor for and activates the effector functions of granulocytes, monocytes/macrophages, and eosinophils (1, 2). GM‑CSF promotes a Th1 biased immune response, angiogenesis, allergic inflammation, and the development of autoimmunity (3‑5). It shows clinical effectiveness in ameliorating chemotherapy‑induced neutropenia, and GM‑CSF transfected tumor cells are utilized as cancer vaccines (6, 7). The 22 kDa glycosylated GM‑CSF, similar to IL‑3 and IL‑5, is a cytokine with a core of four bundled alpha ‑helices (8‑12). Mature human GM‑CSF shares 63%‑70% amino acid sequence identity with canine, feline, porcine, and rat GM‑CSF and 54% with mouse GM‑CSF. GM‑CSF exerts its biological effects through a heterodimeric receptor complex composed of GM‑CSF R alpha /CD116 and the signal transducing common beta chain (CD131) which is also a component of the high‑affinity receptors for IL‑3 and IL‑5 (13, 14). In addition, GM‑CSF binds a naturally occurring soluble form of GM‑CSF R alpha (15). Human GM‑CSF is active on canine and feline cells but not on murine cells (16‑18).
- Martinez-Moczygemba, M. and D.P. Huston (2003) J. Allergy Clin. Immunol. 112:653.
- Barreda, D.R. et al. (2004) Dev. Comp. Immunol. 28:509.
- Eksioglu, E.A. et al. (2007) Exp. Hematol. 35:1163.
- Cao, Y. (2007) J. Clin. Invest. 117:2362.
- Fleetwood, A.J. et al. (2005) Crit. Rev. Immunol. 25:405.
- Heuser, M. et al. (2007) Semin. Hematol. 44:148.
- Hege, K.M. et al. (2006) Int. Rev. Immunol. 25:321.
- Kaushansky, K. et al. (1992) Biochemistry 31:1881.
- Diederichs, K. et al. (1991) Science 254:1779.
- Cantrell, M.A. et al. (1985) Proc. Natl. Acad. Sci. 82:6250.
- Lee, F. et al. (1985) Proc. Natl. Acad. Sci. 82:4360.
- Wong, G.G. et al. (1985) Science 228:810.
- Onetto-Pothier, N. et al. (1990) Blood 75:59.
- Hayashida, K. et al. (1990) Proc. Natl. Acad. Sci. 87:9655.
- Pelley, J.L. et al. (2007) Exp. Hematol. 35:1483.
- Hogge, G.S. et al. (1990) Cancer Gene Ther. 6:26.
- Sprague, W.S. et al. (2005) J. Comp. Pathol. 133:136.
- Shanafelt, A.B. et al. (1991) J. Biol. Chem. 266:13804.







 危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)
危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)  营业执照(三证合一)
营业执照(三证合一)