


 下载产品说明书
下载产品说明书 下载SDS
下载SDS 用小程序,查商品更便捷
用小程序,查商品更便捷



 收藏
收藏
 对比
对比 咨询
咨询





参考图片
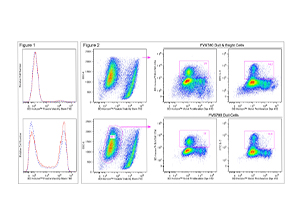
Figure 1. Fluorescent staining of Jurkat cells with BD Horizon™ Fixable Viability Stain 780. Human Jurkat cells were treated (16 hr) with 0.025% DMSO (Top Plot) or 5 μM camptothecin (Bottom Plot) and stained with BD Horizon™ Fixable Viability Stain 780 (Cat. No. 565388). Cells were either not fixed (solid line histograms), or fixed in BD Cytofix™ Fixation Buffer (Cat. No. 554655) and permeabilized in Perm/Wash Buffer I (Cat. No. 557885) (dashed line histograms). Histograms were derived from gated events with the light scattering characteristics of Jurkat cells. Flow cytometry was performed using a BD™ LSRII Cell Analyzer System. Figure 2. Analysis of proliferating mouse splenocytes for surface and intracellular markers. BALB/c splenocytes were stained (10 min, 37°C) with BD Horizon™ Violet Proliferation Dye 450 (Cat. No. 562158), washed twice, and then cultured (3 days) with Purified NA/LE Hamster Anti-Mouse CD3e (Cat. No. 553057) and Hamster Anti-Mouse CD28 (Cat. No. 553294) antibodies. The cells were restimulated (4 hr) with PMA, Ionomycin, and BD GolgiStop™ Protein Transport Inhibitor (Monensin) (Cat. No. 554724). Cells were harvested, stained with BD Horizon™ Fixable Viability Stain 780, fixed and permeabilized using a BD Cytofix/Cytoperm™ Fixation/Permeabilization Solution Kit (Cat. No. 554714), and then stained with BD Horizon™ BUV395 Anti-Mouse CD4 (Cat. No. 563790) and FITC Anti-Mouse IL-2 (Cat. No. 554427) antibodies. Two-color dot plots showing VPD450 fluorescence versus CD4 expression (Middle Plots) or IL-2 expression (Right Plots) were derived from either total cells (Top; FVS780 Dull and Bright Cells) or previously viable cells (Bottom; FVS780 Dull Cells) with the light scatter characteristics of intact cells. CD4+ and IL-2+ cell gates were based on an FMO or unstimulated cell control, respectively. Flow cytometry was performed on a BD LSRFortessa™ Cell Analyzer system.






 危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)
危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)  营业执照(三证合一)
营业执照(三证合一)