

Product Usage Information
1. Prepare the following reagents with reverse osmosis deionized (RODI) or equivalent grade water:a. 1X PBS (azide- and protein/serum-free)b. Incubation Buffer: Dissolve 0.5 g Bovine Serum Albumin (BSA) (#9998) in 100 ml 1X PBS. Store at 4°C.2. Remove Ghost Dye™ from -20°C and bring to room temperature.3. Collect cells by centrifugation and aspirate supernatant.4. Wash cells by centrifugation in excess 1X PBS. Repeat if necessary.5. Resuspend cells to a concentration of 1-10 x 106/mL in 1X PBS.6. Centrifuge the Ghost Dye™ before opening then add 1 uL for each 1 mL of cell suspension and vortex immediately.7. Incubate for 30 minutes at 4°C protected from light.8. Wash by centrifugation in excess incubation buffer. Discard supernatant. Repeat.9. Cells can then be fixed, permeabilized, and immunostained based upon experimental design and recommended protocols.10. Exclude cells with high Ghost Dye™ fluorescence from analysis. These were non-viable cells at the time of fixation. See details below for excitation and emission specifications. Ghost Dye™ Red 710 Fixable Viability Dye excited by the red (633-647 nm) laser line and has a peak emission of 710 nm that can be detected using the recommended 710/50 band pass filter commonly used for detection of Alexa Fluor® 700.




参考图片
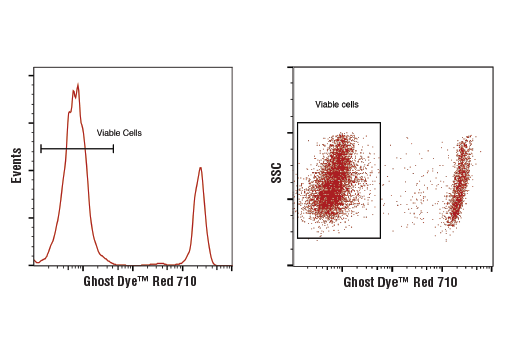
Flow cytometric analysis of live and fixed/permeabilized peripheral blood mononuclear cells, combined and stained with Ghost Dye™ Red 710 Viability Dye. Viable gate is indicated.








 用小程序,查商品更便捷
用小程序,查商品更便捷







 危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)
危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)  营业执照(三证合一)
营业执照(三证合一)