


 下载产品说明书
下载产品说明书 下载SDS
下载SDS 用小程序,查商品更便捷
用小程序,查商品更便捷



 收藏
收藏
 对比
对比 咨询
咨询Simple Western(5 µg/mL)
Immunohistochemistry(5-15 µg/mL)
Immunocytochemistry(5-15 µg/mL)
Simple Western(5 µg/mL)
Immunohistochemistry(5-15 µg/mL)
Immunocytochemistry(5-15 µg/mL)




Met16-Thr718
Accession # P11247

Scientific Data
 View Larger
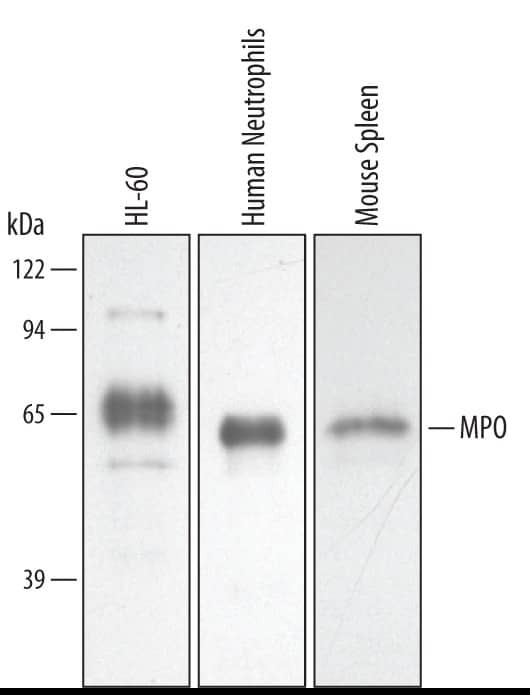
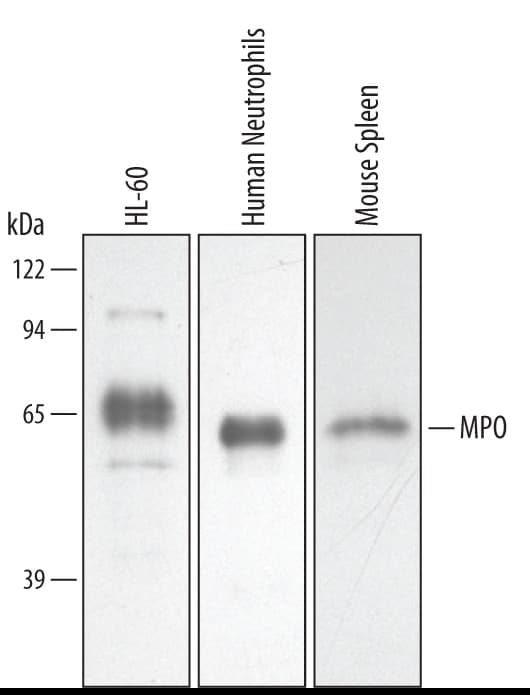
View LargerDetection of Human/Mouse Myeloperoxidase/MPO by Western Blot. Western blot shows lysates of HL-60 human acute promyelocytic leukemia cell line, human neutrophil cells, and mouse spleen tissue. PVDF membrane was probed with 0.5 µg/mL of Goat Anti-Human/Mouse Myeloperoxidase/MPO Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3667) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (HAF019). A specific band was detected for Myeloperoxidase/MPO at approximately 60 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 2.
 View Larger
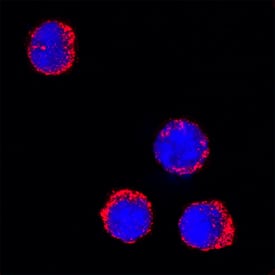
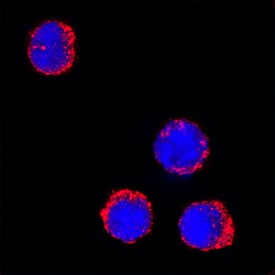
View LargerMyeloperoxidase/MPO in Mouse Splenocytes. Myeloperoxidase/MPO was detected in immersion fixed mouse splenocytes using Goat Anti-Human/Mouse Myeloperoxidase/MPO Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3667) at 15 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (red; NL001) and counterstained with DAPI (blue). Specific staining was localized to cytoplasm. View our protocol for Fluorescent ICC Staining of Non-adherent Cells.
 View Larger
View LargerMyeloperoxidase/MPO in Mouse Spleen Tissue. Myeloperoxidase/MPO was detected in immersion fixed paraffin-embedded sections of mouse spleen tissue using Goat Anti-Human/Mouse Myeloperoxidase/MPO Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3667) at 1 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Goat IgG VisUCyte™ HRP Polymer Antibody (VC004). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to lymphocytes. Staining was performed using our IHC Staining with VisUCyte HRP Polymer Detection Reagents.
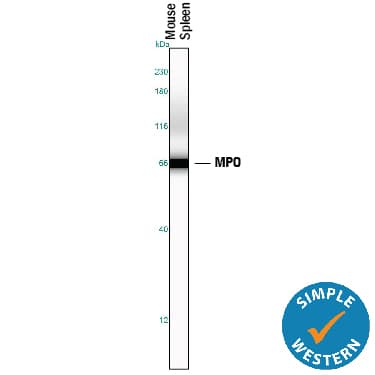
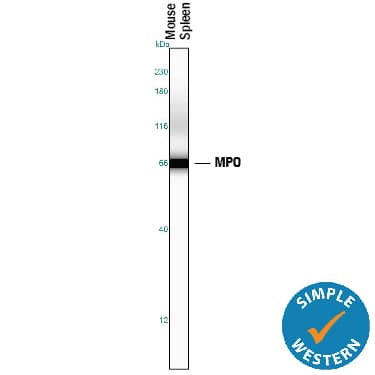 View Larger
View LargerDetection of Mouse Myeloperoxidase/MPO by Simple WesternTM. Simple Western lane view shows lysates of mouse spleen tissue, loaded at 0.2 mg/mL. A specific band was detected for Myeloperoxidase/MPO at approximately 67 kDa (as indicated) using 5 µg/mL of Goat Anti-Human/Mouse Myeloperoxidase/MPO Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3667) followed by 1:50 dilution of HRP-conjugated Anti-Goat IgG Secondary Antibody (HAF109). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
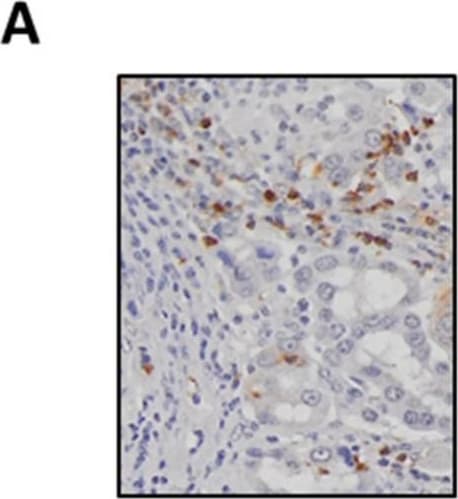
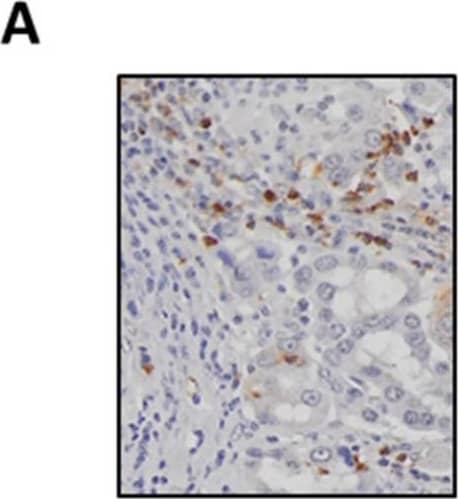 View Larger
View LargerDetection of Human Myeloperoxidase/MPO by Immunohistochemistry SAA levels positively correlate with tumour-associated neutrophil infiltration. (A) Representative images of MPO-positive neutrophils in a lung adenocarcinoma tumour section (original magnification ×200). (B) Neutrophils are significantly elevated in tumours compared to control tissue biopsies, which was confirmed by (C) paired analysis of tumour and adjacent control lung tissue. (D) Circulating SAA levels were elevated in lung adenocarcinoma patients as determined by ELISA. (E) SAA transcript levels were not increased in tumours as measured by RTqPCR and confirmed by (F) sub-analysis of the paired from the tumour and adjacent control tissue samples. (G) Tissue sections were stained for SAA, which identified positive staining within seromucinous glands and tumour-associated macrophages (original magnification ×200). (H) Spearman correlation was used to assess associations between SAA transcript levels and tumour infiltrating neutrophil density (r = 0.51, *** p < 0.001). **** p < 0.0001, ** p < 0.01, ## p < 0.01. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/34203378), licensed under a CC-BY license. Not internally tested by R&D Systems.
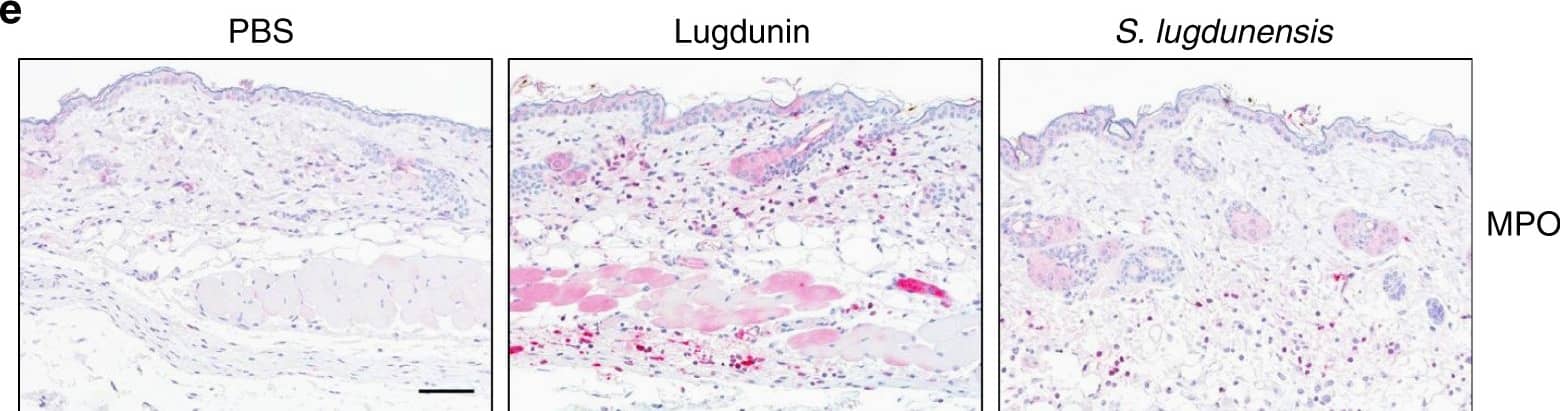 View Larger
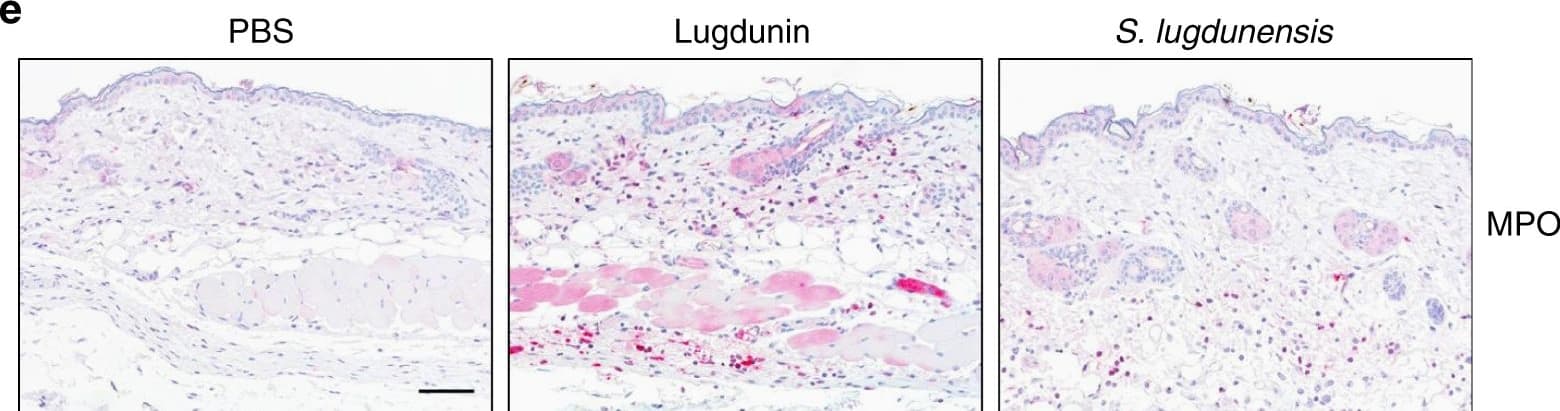
View LargerDetection of Mouse Myeloperoxidase/MPO by Immunocytochemistry/Immunofluorescence Epicutaneous lugdunin recruits phagocytic cells. a Schematic overview of the mouse experiments: 6–8-week-old female C5BL/6 wild-type (WT), MyD88-ko, or 5xTLR-ko mice were epicutaneously treated with 1.5 µg lugdunin or phosphate-buffered saline (PBS) as a control. After 24 h, mice were euthanized, immune cells were isolated from treated skin areas, and immune cell composition was analyzed by flow cytometry. b Shown is the mean percentage of CD45+ live cells in mouse skin of 10 C57BL/6 WT mice ± s.e.m. One mouse is represented as two dots analyzed by two different stainings. c Pie charts show the mean percentage of the different immune cell subsets in the skin of 10 WT mice after 24 h of PBS or lugdunin treatment. d Shown are representative flow cytometry data (left panel) and the mean percentage of neutrophils (Ly6C+Ly6G+) and monocytes (Ly6C+Ly6G−) pregated on CD11b+CD45+ live cells (see Supplementary Fig. 3a, f for the gating strategy) in mouse skin ± s.e.m. One dot represents one mouse. *P < 0.05. e Representative myeloperoxidase (MPO)-stained paraffin-embedded mouse skin sections. Scale bar, 100 µM. Source data are provided as a Source Data file Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31227691), licensed under a CC-BY license. Not internally tested by R&D Systems.
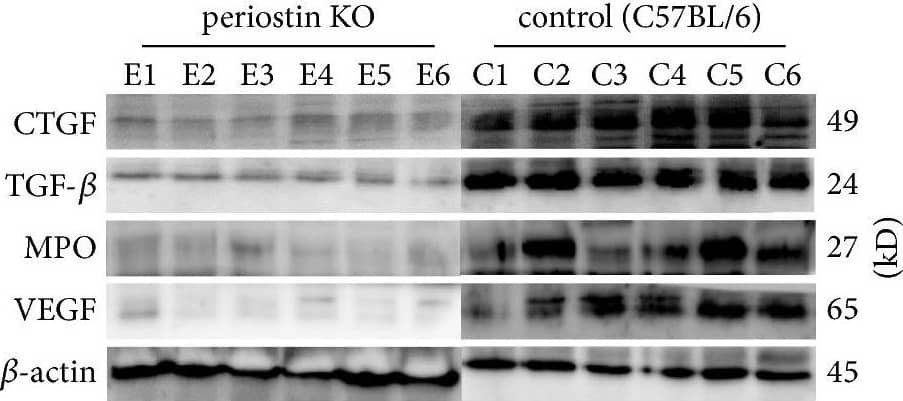 View Larger
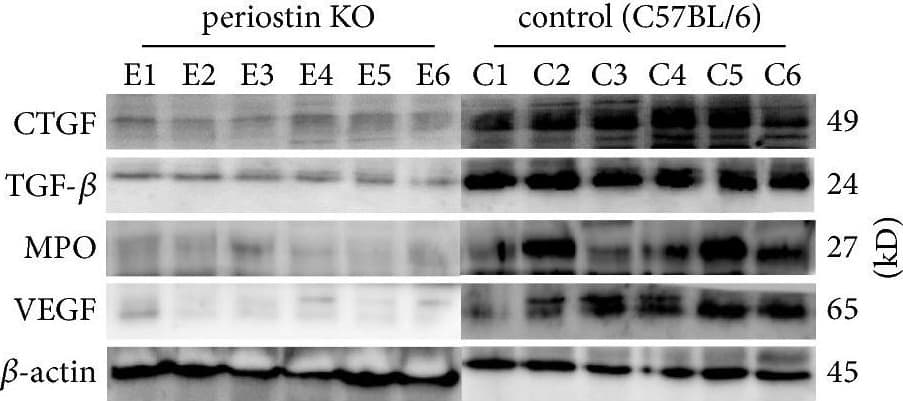
View LargerDetection of Mouse Myeloperoxidase/MPO by Western Blot Levels of CTGF, TGF-beta, MPO, and VEGF in silicone implant-induced capsular tissues determined by western blotting (a). A low signal was obtained for CTGF (b) and TGF-beta (c) protein in PN-KO mice (n = 6), whereas a strong signal was detected in the C57BL/6 mice (n = 6). Compared with the control group (n = 6), the levels of MPO (d) and VEGF (e) protein were downregulated in the PN-KO group (n = 6). Relative expression levels normalized to the housekeeping gene beta -actin. ∗∗p < 0.001. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/29854742), licensed under a CC-BY license. Not internally tested by R&D Systems.
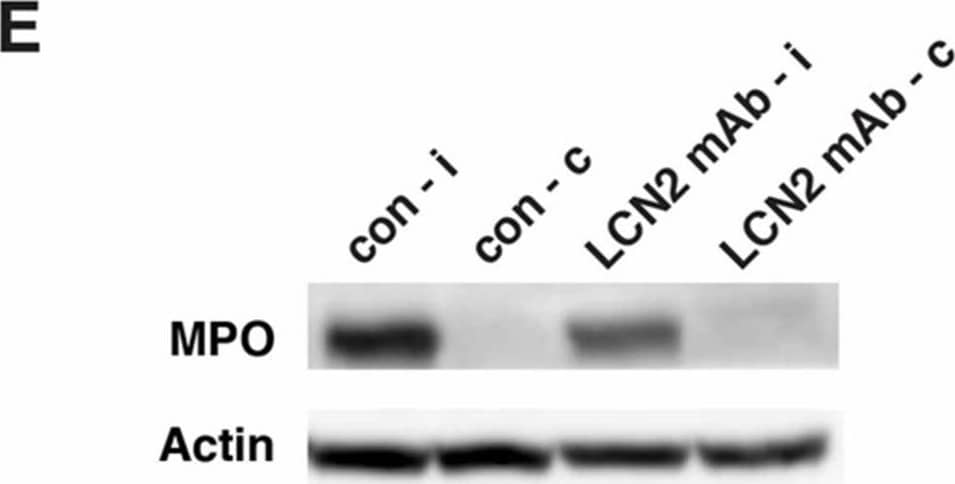 View Larger
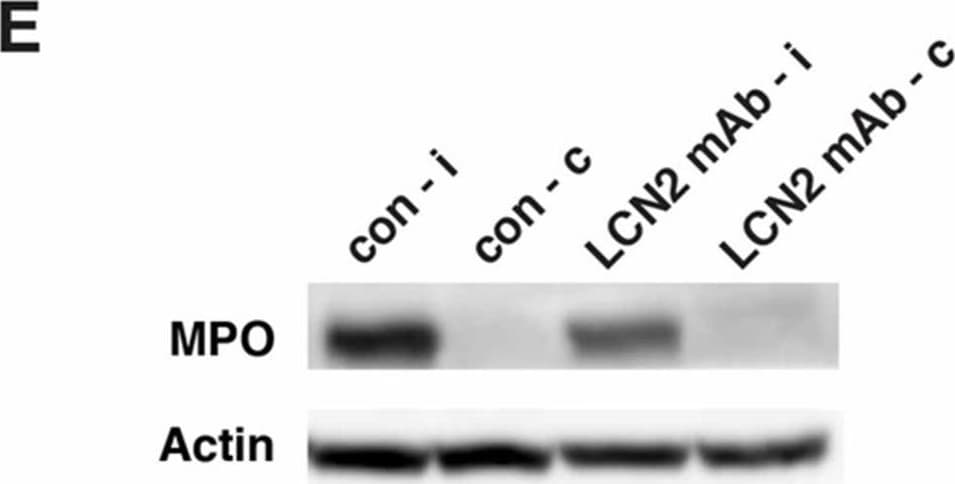
View LargerDetection of Mouse Myeloperoxidase/MPO by Western Blot LCN2 mAb limited blood–brain barrier leakage and infiltration of neutrophils after tMCAo. Representative images (A) and quantification (B) of Evans blue extravasation in the ipsilateral hemispheres of mice treated with control IgG (con) and LCN2 mAb (n = 5 per group) after one hour of tMCAo and 23 h after reperfusion. The concentration of Evans blue dye in the ipsilateral hemispheres of mice treated with LCN2 mAb was significantly decreased (** p < 0.01) as compared with that in the ipsilateral hemispheres of mice treated with control IgG (two-tailed, unpaired t test); (C) The expression level of the tight junction protein claudin-5 was analyzed after treatments with control IgG and LCN2 mAb (n = 4 per group). The ipsilateral (i) and contralateral (c) hemispheres isolated at 23 h after tMCAo were analyzed by Western blotting using antibodies against claudin-5. Representative Western blot showing the expression of claudin-5 (~22 kDa) in brain homogenates. beta -actin served as a loading control; (D) The level of claudin-5 immunoreactivity normalized to beta -actin (claudin-5/actin) in the ipsilateral hemispheres in mice treated with LCN2 mAb was significantly higher than that in the ipsilateral hemispheres of mice that received the control IgG (* p < 0.05, one-tailed, unpaired t test); (E,F) Neutrophil infiltration was analyzed by measuring the levels of MPO in brain homogenates. The ipsilateral (i) and contralateral (c) hemispheres of mice treated with control IgG (con) and LCN2 mAb (n = 4 per group) isolated at 23 h after tMCAo were analyzed by Western blotting using antibodies against MPO; (E) Representative Western blots show the expression of MPO heavy chain (~55 kD) in brain homogenates; (F) The level of MPO immunoreactivity normalized to beta -actin (MPO/actin) was significantly reduced in the ipsilateral hemispheres of mice treated with LCN2 mAb (* p < 0.05, one-tailed, unpaired t test) as compared with that in the ipsilateral hemisphere of mice that received control IgG. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32872405), licensed under a CC-BY license. Not internally tested by R&D Systems.
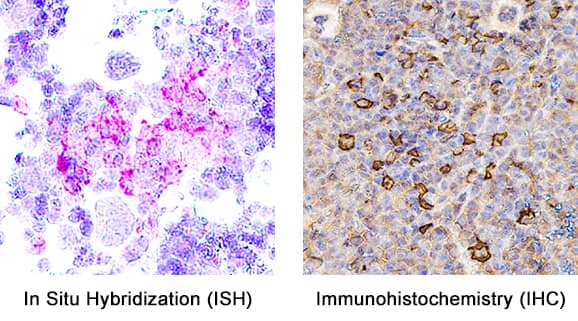 View Larger
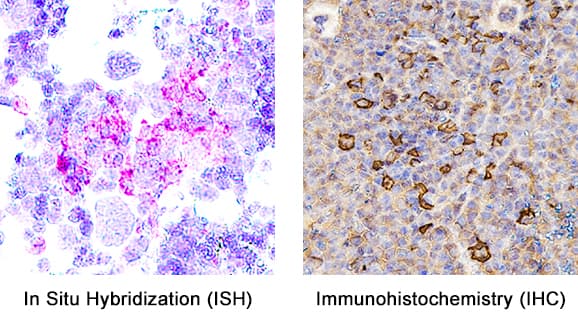
View LargerDetection of Myeloperoxidase/MPO in Mouse Spleen. Formalin-fixed paraffin-embedded tissue sections of mouse spleen were probed for MPO mRNA (ACD RNAScope Probe, catalog #603091; Fast Red chromogen, ACD catalog # 322750). Adjacent tissue section was processed for immunohistochemistry using goat anti-mouse MPO polyclonal antibody (R&D Systems catalog # AF3667) at 1ug/mL with overnight incubation at 4 degrees Celsius followed by incubation with anti-goat IgG VisUCyte HRP Polymer Antibody (Catalog # VC004) and DAB chromogen (yellow-brown). Tissue was counterstained with hematoxylin (blue). Specific staining was localized to cell surface.
Human/Mouse Myeloperoxidase/MPO Antibody Summary
Met16-Thr718
Accession # P11247
*Small pack size (-SP) is supplied either lyophilized or as a 0.2 µm filtered solution in PBS.
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Simple Western(5 µg/mL)
Immunohistochemistry(5-15 µg/mL)
Immunocytochemistry(5-15 µg/mL)


Background: Myeloperoxidase/MPO
Myeloperoxidase (MPO) is a hemeprotein that belongs to the XPO subfamily of the heme peroxidase superfamily. MPO is synthesized as a preproprotein that undergoes proteolytic processing to generate a disulfide-linked heterodimer of the N-terminal beta -subunit (12 kDa) and C-terminal alpha subunit (60 kDa). Active MPO is a tetramer of two beta -subunits and two alpha -subunits that are also disulfide-linked through the two alpha -subunits. MPO is stored in granules and is an abundant protein in neutrophils and monocytes. MPO is released upon activation to catalyze the formation of powerful oxidants such as hypochlorous acid, which kills microbes. Unprocessed pro-MPO can also be released. Mouse MPO shares 87% amino acid sequence identity with that of human MPO.


Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
参考图片
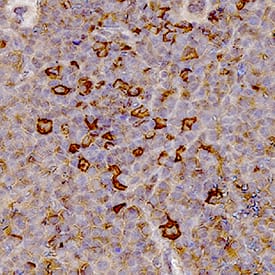
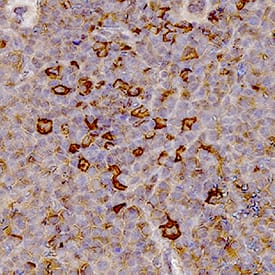
Myeloperoxidase/MPO in Human Spleen.
Detection of Human/Mouse Myeloperoxidase/MPO by Western Blot.
Detection of Mouse Myeloperoxidase/MPO by Simple WesternTM.
12-230 kDa separation system.
Myeloperoxidase/MPO in Mouse Splenocytes.





 危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)
危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)  营业执照(三证合一)
营业执照(三证合一)