
 下载产品说明书
下载产品说明书 用小程序,查商品更便捷
用小程序,查商品更便捷



 收藏
收藏
 对比
对比 咨询
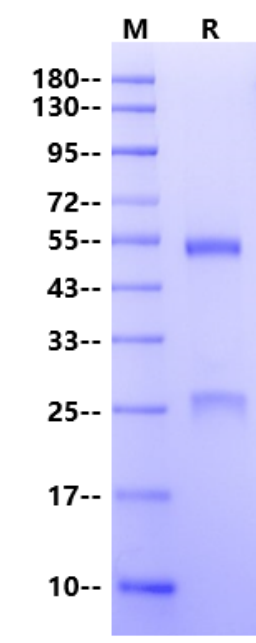
咨询27 kDa (Light Chain) & 54 kDa (Heavy Chain) (Reducing)
27 kDa (Light Chain) & 54 kDa (Heavy Chain) (Reducing)

27 kDa (Light Chain) & 54 kDa (Heavy Chain) (Reducing)







2μg (R: reducing condition).
"}]

Atopic dermatitis (AD) is a common inflammatory skin disease that has emerging treatments targeting the underlying immunological mechanism. Interleukin-31 (IL-31) is associated with the pathobiological mechanism of AD, contributing to symptoms such as dermatitis and pruritus. Nemolizumab is an anti-IL-31 receptor α-chain (IL-31RA) monoclonal antibody agent that is efficacious in improving symptoms of AD in several phase II and phase III studies in recent years. Nemolizumab demonstrates great efficacy in reducing pruritus and to a lesser degree, dermatitis associated with AD. Additionally, one advantage of nemolizumab is its quick speed of action. Adverse effects are mild and transient in nature, including exacerbation of AD, nasopharyngitis, upper respiratory tract infections, elevated creatine kinase and peripheral edema. Severe adverse effects were not common and consisted of exacerbation of AD and asthma exacerbation. Therefore, nemolizumab has the potential to be an important treatment of choice for AD given its efficacy, mild side effect profile and rapid time of onset.

Reconstitute at 0.1-1 mg/ml according to the size in ultrapure water after rapid centrifugation.

·12 months from date of receipt, -20 to -70 °C as supplied. ·1 month, 2 to 8 °C under sterile conditions after reconstitution. ·Please avoid repeated freeze-thaw cycles.
Angelina Labib, Ashley Vander Does. Nemolizumab for atopic dermatitis. Drugs Today (Barc).2022 Apr;58(4):159-173.

参考图片
2μg (R: reducing condition).







 危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)
危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)  营业执照(三证合一)
营业执照(三证合一)