


 下载产品说明书
下载产品说明书 用小程序,查商品更便捷
用小程序,查商品更便捷



 收藏
收藏
 对比
对比 咨询
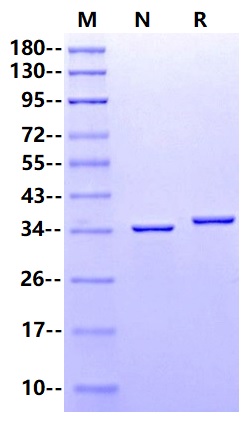
咨询35.7kDa (Reducing)
35.7kDa (Reducing)
>95% by SDS-PAGE
>95% by SDS-PAGE


35.7kDa (Reducing)



>95% by SDS-PAGE




Components | Amount |
PNGase F* | 40000U/ml |
Reaction Buffer (10×) | 200mM Tris, PH 7.5 25°C |
Denaturing Buffer(10×) | 5% SDS、400 mM DTT |
NP-40(10×) | 10% NP-40 in MilliQ-H2O |
* One unit is defined as the amount of enzyme required to remove > 95% of the carbohydrate from 10 μg of denatured RNase B in 1 hour at 37°C in a total reaction volume of 10 μl.





Peptide: N-glycosidase F (PNGase F) is an asparagine amidase produced by Flavobacterium meningosept-icum that serves as a useful tool in the research on protein N-glycosylation. Recombinant expression in E.coli. The cleavage site of PNGase F is the amide bond between N-acetylglucosamine (GlcNAc) and aspartate residues on the medial side of the glycoprotein, and converts aspartyl to aspartic acid on the enzymolysis protein. This product is often used for complete deglycosylation of antibodies and their associated proteins.
The PNGase F Glycan Cleavage Kit includes all components necessary to perform the enzymatic removal of almost all N-linked oligosaccharides from glycoproteins. The kit includes recombinant Peptide N-Glycosidase F(PNGase F) enzyme, which cleaves N-glycan chains at the innermost GlcNAc and asparagine residues of high mannose, hybrid, and complex oligosaccharides, and a 10X reaction buffer.

· 12 months from date of receipt, -20 to -70 °C as supplied.
· Please avoid repeated freeze-thaw cycles.
1. Frank Maley and Robert B. Trimble and Anthony L. Tarentino and Thomas H. Plummer Jr. Characterization of glycoproteins and their associated oligosaccharides through the use of endoglycosidases[J]. Analytical Biochemistry, 1989.

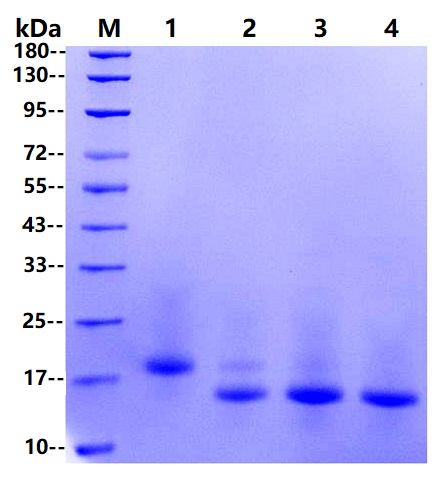
参考图片
M marker
1: Rnase B 10μg
2: Rnase B 10μg+0.25U PNGF
3: Rnase B 10μg+0.5U PNGF
4: Rnase B 10μg+1U PNGF
Complement PNGaseF,1µg on SDS-PAGE under reducing and Non-reducing condition. The Purity is greater than 95%.





 危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)
危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)  营业执照(三证合一)
营业执照(三证合一)