



 下载产品说明书
下载产品说明书 用小程序,查商品更便捷
用小程序,查商品更便捷



 收藏
收藏
 对比
对比 咨询
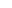
咨询27 kDa (Reducing)
27 kDa (Reducing)
>95% by SDS-PAGE and RP-HPLC
>95% by SDS-PAGE and RP-HPLC

27 kDa (Reducing)



>95% by SDS-PAGE and RP-HPLC





1. SUMO Protease;
2. 10X SUMO Protease Buffer + Salt: 500 mM PB, pH 7.4, 2% Igepal (NP-40), 1.5 M NaCl, 10 mM DTT;
3. 10X SUMO Protease Buffer – Salt: 500 mM PB, pH 7.4, 2% Igepal (NP-40), 10 mM DTT;




Saccharomyces cerevisiae-derived Small ubiquitin-like modifier (SUMO, Smt3) is commonly used as a protein fusion domain to facilitate expression and purification of recombinant proteins, and a Saccharomyces cerevisiae-derived SUMO-specific protease(Ulp1) is then used to remove SUMO tag from these proteins in a ‘scarless’ manner. SUMO Protease cleaves in a highly specific manner, recognizing the tertiary structure of the SUMO tag, rather than an amino acid sequence, and hydrolyzes the peptide bond in the x-Gly-Gly-x sequence after the Gly-Gly bond at the C-terminus of the SUMO tag. The SUMO Protease cleavage proteins over wide ranges of temperature (4℃-30℃), ionic strengths(0-400 mM NaCl) and pH(7.0-9.0), and easily removed from the cleavage reaction by Immobilized Metal Affinity chromatography (IMAC).

· 12 months from date of receipt, -20 to -70 °C as supplied.
· 6 months, -20 to -70 °C under sterile conditions after reconstitution.
· 1 week, 2 to 8 °C under sterile conditions after reconstitution.
· Please avoid repeated freeze-thaw cycles.
参考图片
The substrate of 2 μg fusion protein was digested by SUMO Protease at 30 ℃ for 1 hour.
The addition amount of SUMO Protease were 0U, 0.25U, 0.5U, 0.75U and 1U, respectively.
The substrate is a fusion protein with a molecular weight of 20 KD and digested completely into two bands.
1μg (N: non-reducing condition, R: reducing condition).





 危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)
危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)  营业执照(三证合一)
营业执照(三证合一)