视频素材来源于JOVE https://www.jove.com/cn/v/50765/isolation-and-th17-differentiation-of-naive-cd4-t-lymphocytes
版权归原作者所有
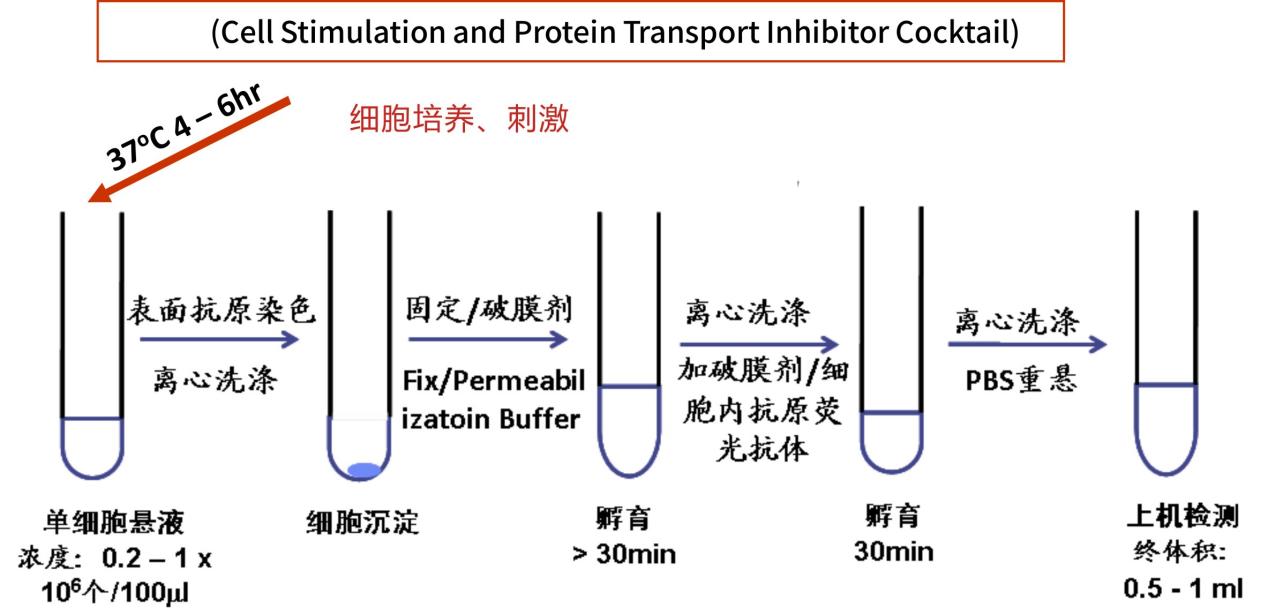
实验步骤
- 1
- 2
-
人外周血TH1 /TH2/TH17流式检测实验方案—分离PBMC法
实验所需设备: 流式上样管,6孔细胞培养板,37℃孵箱,5%CO2。混匀振荡器,掌上离心机,水平转子离心机,流式细胞仪
实验所需试剂: 肝素钠抗凝的全血(BD 肝素钠抗凝管),RPMI-1640,FBS,淋巴细胞分离液,普通PBS
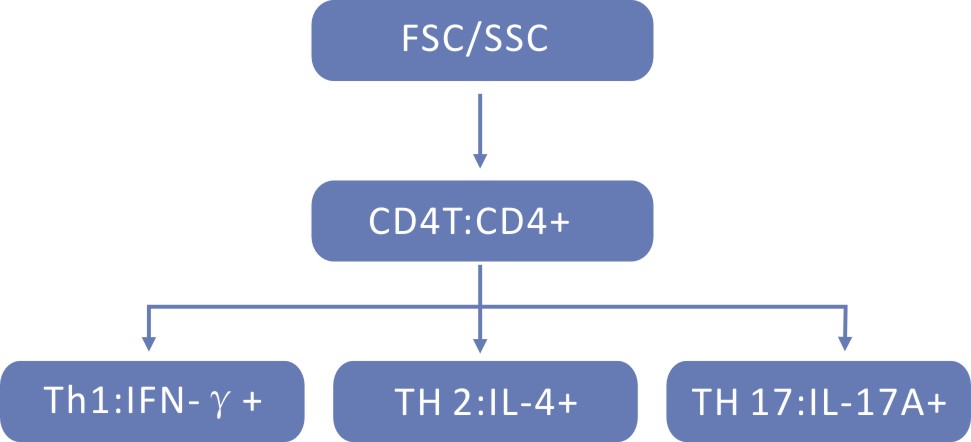
MA/Ionomycin + BFA/Monensin
△点击放大图片
△点击放大图片
-
实验步骤
PBMC的分离
1. 4ml新鲜抗凝血,用人外周血淋巴细胞分离液分离出PBMC(参考分离液产品说明书)。
2. 吸取PBMC细胞层。PBMC层在血浆下面。小心吸取,吸到15ml离心管中。
3. RPMI 1640洗涤一次。加RPMI 1640至8ml体积,颠倒混匀,离心, 20℃,250g,10min。
4. 弃上清,重悬细胞到3 ml RPMI 1640(含10%FBS)。细胞计数。备用。
PBMC刺激
5. 取出6孔板,做好标记(未刺激孔,刺激孔),每孔加入1mlPBMC, 再分别加入1ml RPMI1640(10%FBS),混匀,避免产生气泡。
6. 刺激孔加入刺激剂(550583)4ul,用枪吹打混匀,尽量避免产生气泡。
7. 放入37℃,5%CO2培养箱培养5h。
8. 5小时后,收细胞,350g,离心,5 minutes,弃上清。
9. 刺激5小时后,收细胞,同时加入1ml RPMI1640冲洗细胞培养板,一起收集到离心管中,350g,离心,5 minutes,弃上清。
10. 分别加入2ml stainbuffer中,离心,350g,5 minutes。
11. 弃上清,分别重悬细胞到1mlstainbuffer/管。
阻断,表面染色
12. 分别按照未刺激组和刺激组分管,每管100ul细胞悬液。
13. 每管中分别加入2.5 ug 的Human Fc阻断剂,混匀并室温孵育10min。
14. 分别加入相应的表面标记抗体,混匀后,室温避光孵育15min。
15. 每管中加入2ml 的stain buffer重悬细胞,350g离心5min.
固定破膜 胞内染色
16.用100ul PermWashBuffer重悬固定破膜后的细胞,按照管设置对应加入IL-4,IFN-r,室温避光孵育20min。
17. 用stain buffer洗细胞两次。350g离心5 minutes,弃上清。
18.用0.3ml StainBuffer重悬固定染色后的细胞,上机检测分析。
Human 外周血Th1/Th2/Th17胞内流式检测案例流式图: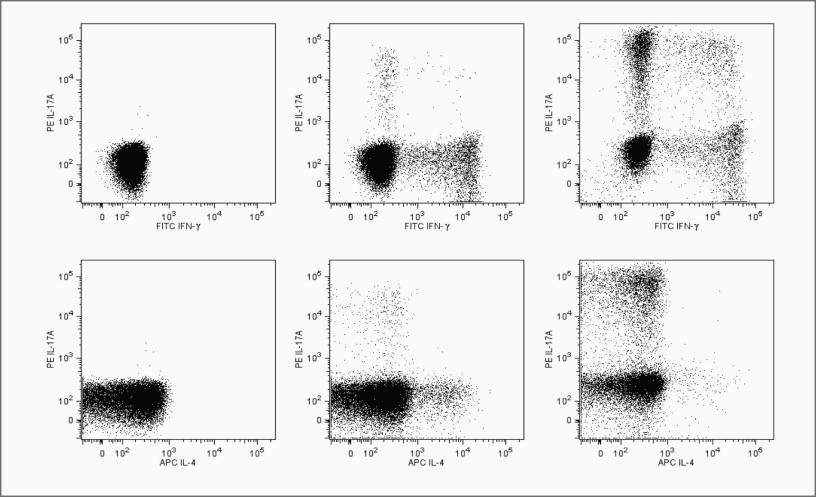
△点击放大图片
Flow cytometric analysis of peripheral blood mononuclear cells (PBMC) using the Human Th1/Th2/Th17 Phenotyping Kit.
The staining pattern of IFN-γ, IL-17A and IL-4 on resting PBMC (left column), PMA/Ionomycin stimulated PBMC (middle column) and polarized Th17 cells (right column) are shown. The top three panels show the staining of IL-17A vs. IFN-γ and the bottom three panels show the staining of IL-17A vs. IL-4. Dot plot analyses are derived from gated CD4+ cell populations. Flow cytometry was performed on a BD™LSR II
Mouse脾脏 Th1/Th2/Th17胞内流式检测案例流式图: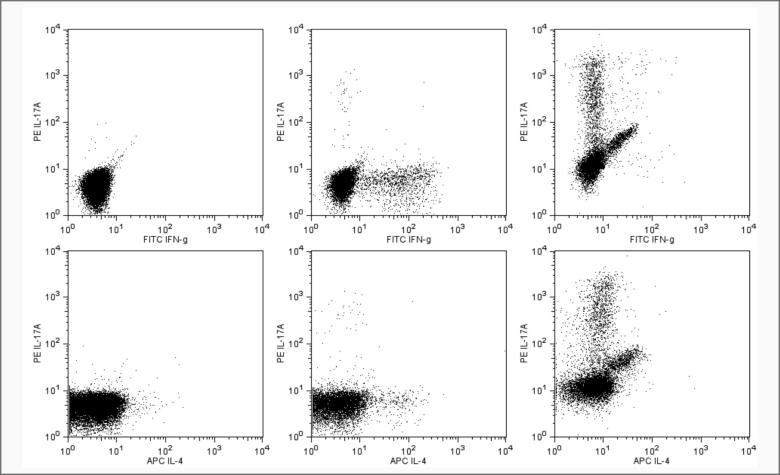
△点击放大图片
Flow cytometric analysis of Mouse Th1/Th2/Th17 phenotyping kit. The staining pattern of IFN-g, IL-17A and IL-4 on resting spleen cells(left column), PMA/Ionomycin stimulated spleen cells (middle column) and polarized Th17 cells (right column) are shown. The top three panels show the staining of IL-17A vs. IFN-γ and bottom three panels show the staining of IL-17A vs IL-4. Th17 cells were generated from mouse spleen cells for a 14 days period under polarizing condition described by Chen Dong et al (see references below). Dead cells appear on the diagonal. Dot plot analyses are derived from gated CD4+ cell populations. Flow cytometry was performed on a BD FACSCalibur™.System.


