


 下载产品说明书
下载产品说明书 下载SDS
下载SDS 用小程序,查商品更便捷
用小程序,查商品更便捷



 收藏
收藏
 对比
对比 咨询
咨询Scientific Data
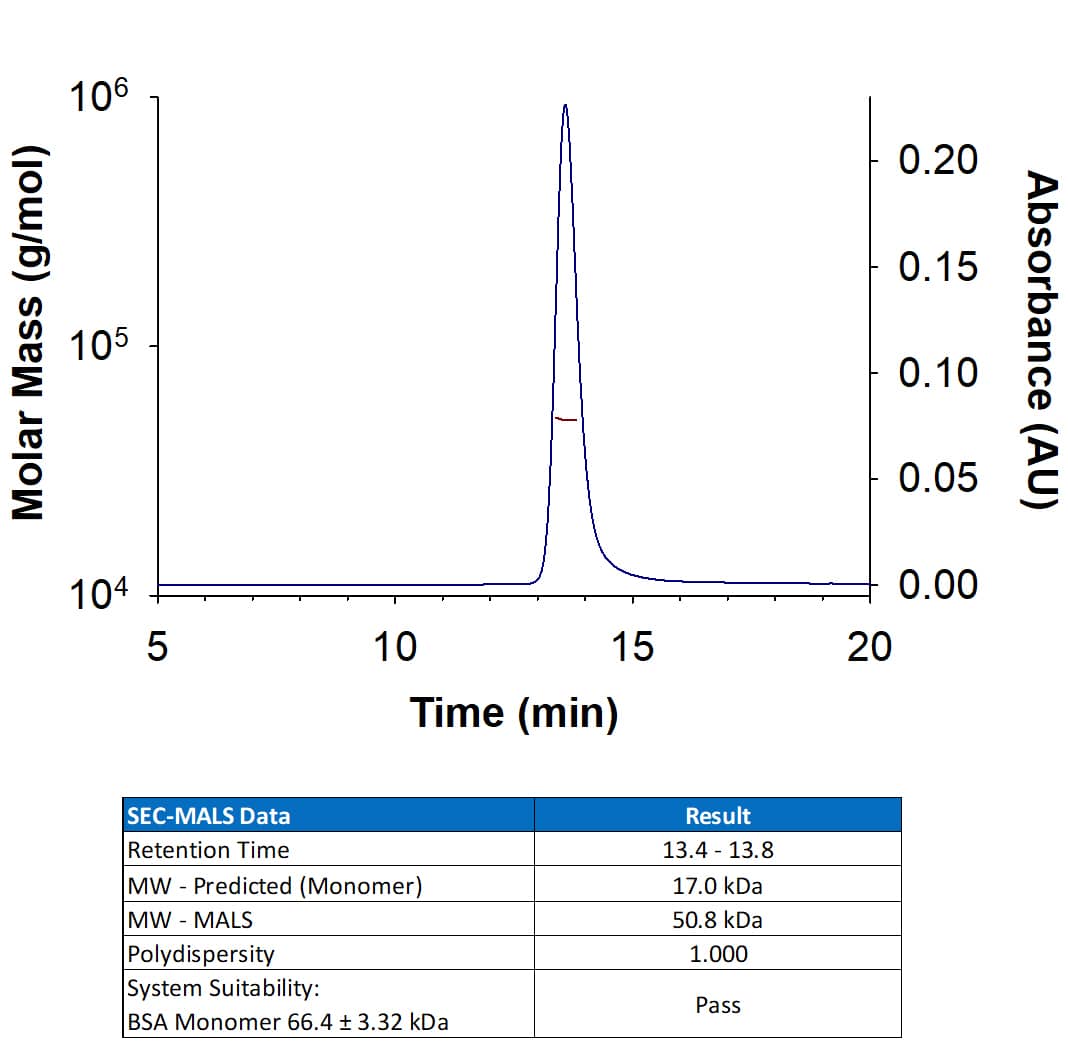 View Larger
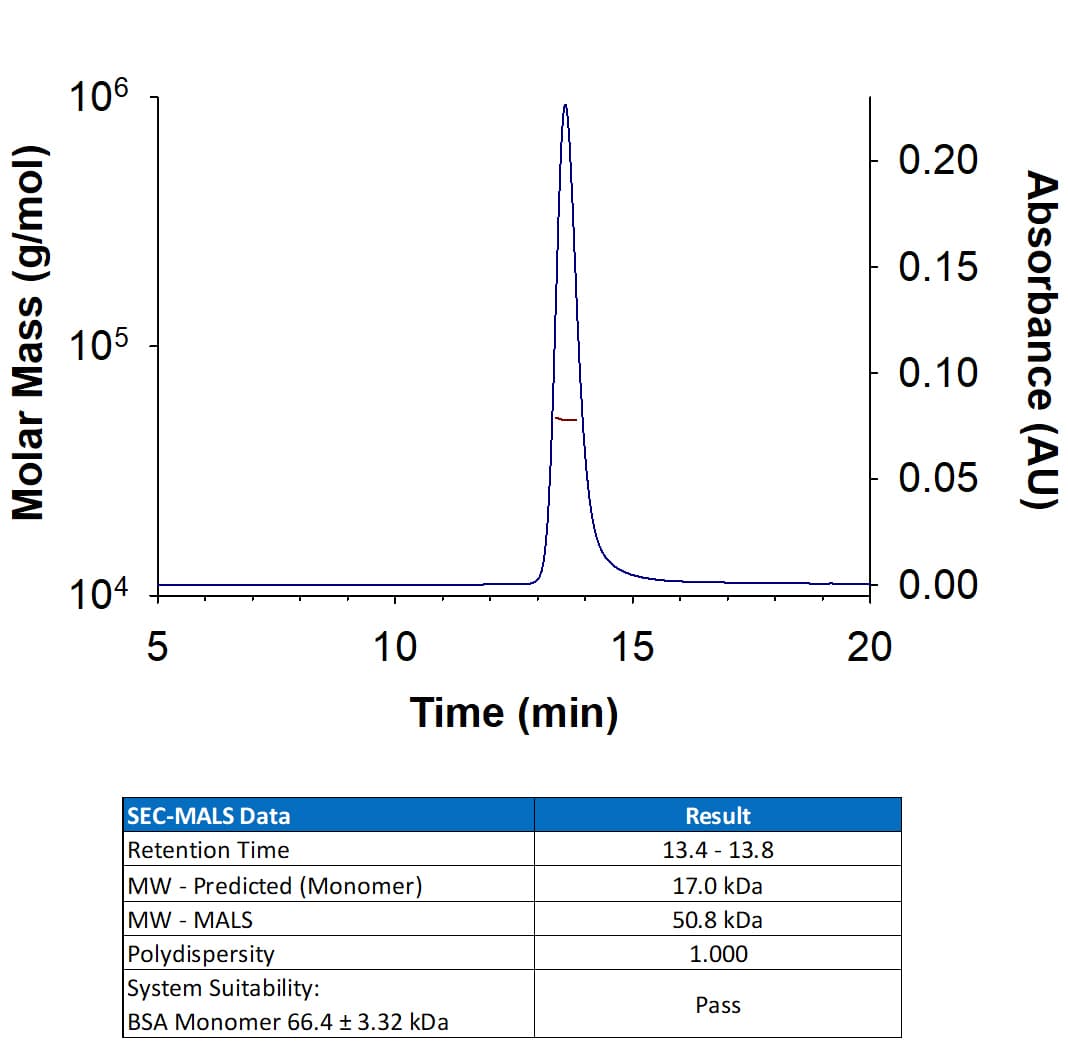
View LargerRecombinant Mouse TNF‑ alpha Protein (Catalog # 410-MT) has a molecular weight (MW) of 50.8 kDa as analyzed by SEC-MALS, suggesting that this protein is a homotrimer.
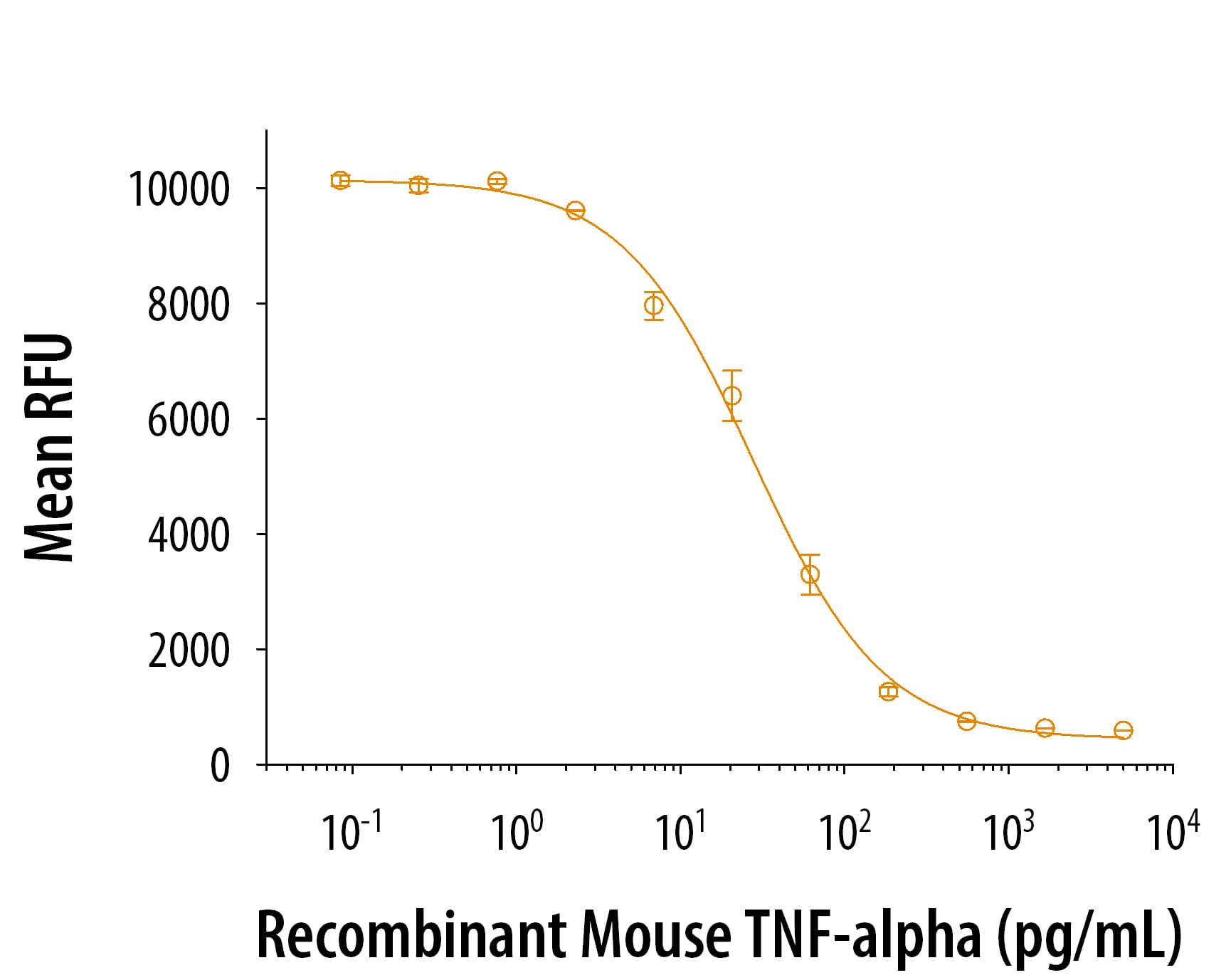
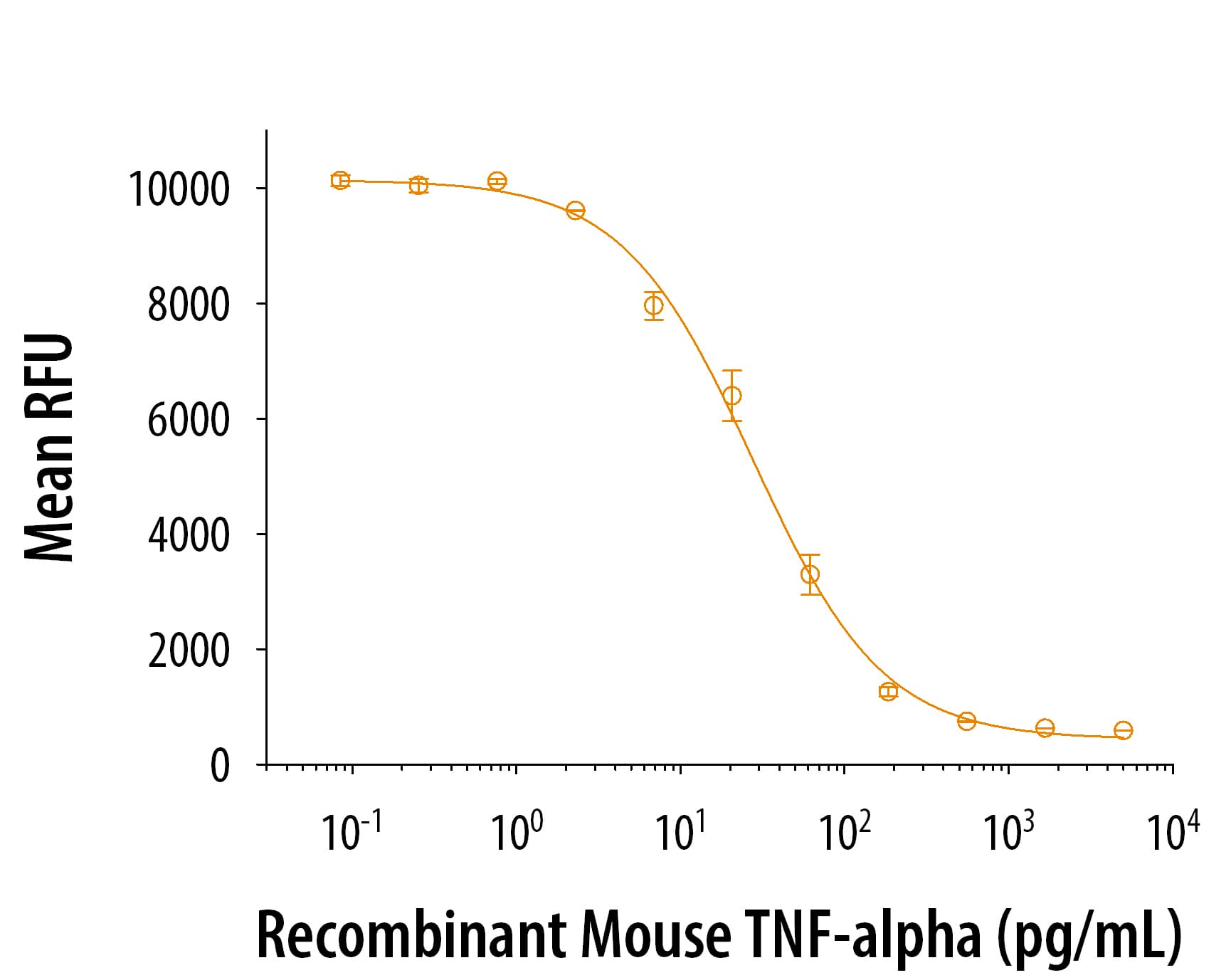 View Larger
View LargerRecombinant mouse TNF-alpha (410-MT) induces cytotoxicity in the L-929 mouse fibroblast cell line in the presence of the metabolic inhibitor actinomycin D. The ED50 for this effect is 8-50 pg/mL.
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
410-MT
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with BSA as a carrier protein. |
| Reconstitution | Reconstitute at 100 μg/mL in sterile PBS containing at least 0.1% human or bovine serum albumin. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
410-MT/CF
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS. |
| Reconstitution | Reconstitute at 100 μg/mL in sterile PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Recombinant Mouse TNF-alpha (aa 80-235) Protein Summary
Product Specifications
Leu80-Leu235, with an N-terminal Met
Analysis
Background: TNF-alpha
Tumor necrosis factor alpha (TNF-alpha ), also known as cachectin and TNFSF2, is the prototypic ligand of the TNF superfamily. It is a pleiotropic molecule that plays a central role in inflammation, immune system development, apoptosis, and lipid metabolism (1, 2). Mouse TNF-alpha consisits of a 35 amino acid (aa) cytoplasmic domain, a 21 aa transmembrane segment, and a 179 aa extracellular domain (ECD) (3). Within the ECD, mouse TNF-alpha shares 94% aa sequence identity with rat and 70%-77% with bovine, canine, cotton rat, equine, feline, human, porcine, rat, and rhesus TNF-alpha. TNF-alpha is produced by a wide variety of immune, epithelial, endothelial, and tumor cells (1, 2). TNF-alpha is assembled intracellularly to form a noncovalently linked homotrimer which is expressed on the cell surface (4). Cell surface TNF-alpha can induce the lysis of neighboring tumor cells and virus infected cells, and it can generate its own downstream cell signaling following ligation by soluble TNFR I (2, 5). Shedding of membrane bound TNF-alpha by TACE/ADAM17 releases the bioactive cytokine, a 55 kDa soluble trimer of the TNF-alpha extracellular domain (6-8). TNF-alpha binds the ubiquitous 55-60 kDa TNF RI (9, 10) and the hematopoietic cell-restricted 80 kDa TNF RII (11, 12), both of which are also expressed as homotrimers (1, 2, 13). Both type I and type II receptors bind TNF-alpha with comparable affinity (14), although only TNF RI contains a cytoplasmic death domain which triggers the activation of apoptosis. Soluble forms of both types of receptors are released and can neutralize the biological activity of TNF-alpha (15).
- Zelova, H. and J. Hosek (2013) Inflamm. Res. 62:641.
- Juhasz, K. et al. (2013) Expert Rev. Clin. Immunol. 9:335.
- Fransen, L. et al. (1985) Nucleic Acids Res. 13:4417.
- Tang, P. et al. (1996) Biochemistry 35:8216.
- Perez, C. et al. (1990) Cell 63:251.
- Black, R.A. et al. (1997) Nature 385:729.
- Moss, M.L. et al. (1997) Nature 385:733.
- Gearing, A.J.H. et al. (1994) Nature 370:555.
- Schall, T.J. et al. (1990) Cell 61:361.
- Loetscher, H. et al. (1990) Cell 61:351.
- Dembic, Z. et al. (1990) Cytokine 2:231.
- Smith, C.A. et al. (1990) Science 248:1019.
- Loetscher, H. et al. (1991) J. Biol. Chem. 266:18324.
- Pinckard, J.K. et al. (1997) J. Biol. Chem. 272:10784.
- Engelmann, H. et al. (1990) J. Biol. Chem. 265:1531.







 危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)
危险品化学品经营许可证(不带存储) 许可证编号:沪(杨)应急管危经许[2022]202944(QY)  营业执照(三证合一)
营业执照(三证合一)